Feb
28
New Design For Lithium Air Battery Capacity Is A Huge Gain
February 28, 2023 | Leave a Comment
Argonne National Laboratory scientists have built and tested for a thousand cycles a new lithium-air battery design. The new design could one day power cars, domestic airplanes, long-haul trucks and more. That’s because the energy storage capacity greatly surpasses that possible with lithium-ion batteries. Many owners of electric cars have wished for a battery pack that could power their vehicle for more than a thousand miles on a single charge.
The research results have been published in the journal Science.
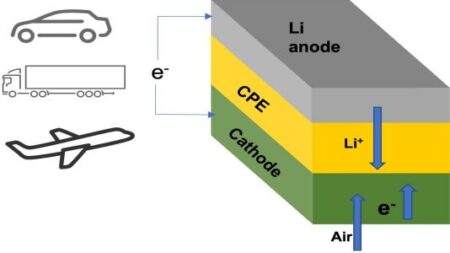
The schematic shows lithium-air battery cell consisting of lithium metal anode, air-based cathode, and solid ceramic polymer electrolyte (CPE). On discharge and charge, lithium ions (Li+) go from anode to cathode, then back. Image Credit: Argonne National Laboratory. Click the press release link for the largest view.
The main new component in this lithium-air battery is a solid electrolyte instead of the usual liquid variety. Batteries with solid electrolytes are not subject to the safety issue with the liquid electrolytes used in lithium-ion and other battery types, which can overheat and catch fire.
More importantly, the team’s battery chemistry with the solid electrolyte can potentially boost the energy density by as much as four times above batteries, which translates into longer driving range.
Larry Curtiss, an Argonne Distinguished Fellow said, “For over a decade, scientists at Argonne and elsewhere have been working overtime to develop a lithium battery that makes use of the oxygen in air. The lithium-air battery has the highest projected energy density of any battery technology being considered for the next generation of batteries beyond lithium-ion.”
In the prior lithium-air designs, the lithium in a lithium metal anode moves through a liquid electrolyte to combine with oxygen during the discharge, yielding lithium peroxide (Li2O2) or superoxide (LiO2) at the cathode. The lithium peroxide or superoxide is then broken back down into its lithium and oxygen components during the charge. This chemical sequence stores and releases energy on demand.
The team’s new solid electrolyte is composed of a ceramic polymer material made from relatively inexpensive elements in nanoparticle form. This new solid enables chemical reactions that produce lithium oxide (Li2O) on discharge.
Argonne chemist Rachid Amine noted that more electrons stored means higher energy density. “The chemical reaction for lithium superoxide or peroxide only involves one or two electrons stored per oxygen molecule, whereas that for lithium oxide involves four electrons,” Amine said.
The team’s lithium-air design is the first lithium-air battery that has achieved a four-electron reaction at room temperature. It also operates with oxygen supplied by air from the surrounding environment. The capability to run with air avoids the need for oxygen tanks to operate, a problem with earlier designs.
The team employed many different techniques to establish that a four-electron reaction was actually taking place. One key technique was transmission electron microscopy (TEM) of the discharge products on the cathode surface, which was carried out at Argonne’s Center for Nanoscale Materials, a DOE Office of Science user facility. The TEM images provided valuable insight into the four-electron discharge mechanism.
Past lithium-air test cells suffered from very short cycle lives. The team established that this shortcoming is not the case for their new battery design by building and operating a test cell for 1000 cycles, demonstrating its stability over repeated charge and discharge.
“With further development, we expect our new design for the lithium-air battery to also reach a record energy density of 1200 watt-hours per kilogram,” said Curtiss. “That is nearly four times better than lithium-ion batteries.”
This research was published in a recent issue of Science. Argonne authors include Larry Curtiss, Rachid Amine, Lei Yu, Jianguo Wen, Tongchao Liu, Hsien-Hau Wang, Paul C. Redfern, Christopher Johnson and Khalil Amine. Authors from IIT include Mohammad Asadi, Mohammadreza Esmaeilirad and Ahmad Mosen Harzandi. And Authors from the University of Illinois Chicago include Reza Shahbazian-Yassar, Mahmoud Tamadoni Saray, Nannan Shan and Anh Ngo.
***
This has to be considered a breakthrough. The big two, capacity and total cycles are covered in a large and impressive way. Plus not hauling along a bottle of O2. There remain the weight and volume questions, but for larger installs like vehicles those questions don’t have the importance like a cell phone or laptop computer would demand. Another prime question would be the temperature range of operation.
As a Department of Energy research effort, access by manufacturers should be easy. Just how a process engineer would view this remains to be seen, but a four fold increase in capacity has to snap attention, investigation and long haul investment considerations.
While the tech isn’t fully ripe just yet, this tech has surely garnered industrial notice. Maybe we’ll see the lithium air battery promotion in the consumers arena sooner rather than later.

