Dec
29
Another Viewpoint Explored For the Sodium Ion Battery
December 29, 2022 | Leave a Comment
A joint research group from Helmholtz-Zentrum Berlin für Materialien und Energie (HZB) and Humboldt-Universität zu Berlin has investigated new combinations of electrolyte solutions and electrode materials for sodium ion battery chemistry. The market for rechargeable batteries is growing rapidly, but the necessary raw materials are limited.
Prof. Philipp Adelhelm, who heads the research group “operando battery analysis,” which was jointly founded by Humboldt University and Helmholtz-Zentrum Berlin in 2020 explained, “In contrast to lithium-ion batteries, which are based on the storage of lithium ions in the positive and negative electrodes of the battery, we are working on the one hand with sodium ions, as they also occur in cheap table salt. On the other hand, we store the sodium ions together with their solvate shell, i.e. solvent molecules from the electrolyte solution that separate the two electrodes. This makes it possible to realize completely new storage reactions.”
The storage of ions when accompanied by their solvation shell in a crystal lattice is referred to as co-intercalation. Up to this point, this concept was limited to the negative electrode of the sodium-ion battery.
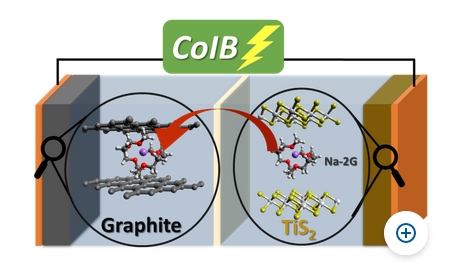
With operando techniques, it is possible to observe how solvated ions embed themselves in batterie electrodes. This might help to develop alternative batteries. Image Credit: © G. A. Ferrero, Helmholtz-Zentrum Berlin für Materialien und Energie. Click the With operando techniques, it is possible to observe how solvated ions embed themselves in batterie electrodes. This might help to develop alternative batteries. Image Credit: © G. A. Ferrero, Helmholtz-Zentrum Berlin für Materialien und Energie. Click the HZB link for more and larger images.
Now the researchers around Adelhelm have succeeded in extending the concept to the positive electrode of the battery.
Dr. Guillermo A. Ferrero, first author of the findings report, explained, “With titanium disulphide and graphite, we have for the first time combined two materials that absorb and release the same solvent during charging and discharging of the battery.”
The scientists could observe changes in the material during charging and discharging via operando measurements performed in the X-Ray Core Lab at HZB on the LIMAX 160. This helped them to assign the co-intercalation mechanism inside the battery. They could then use this new knowledge to realize a battery with two electrodes that both rely on reversible co-intercalation of solvent molecules.
Dr. Katherine A. Mazzio, HZB, explained further, “We are still in the early stages of understanding the implications of the co-intercalation batteries. But there are a few possible advantages we can envision. The process of co-intercalation could improve upon efficiency by enabling better low temperature performance. It could also be utilized to improve upon alternative cell concepts such as using multi-valent ions instead of Li+ or Na+ storage that are particularly sensitive to the solvation shell.”
***
Well, this cracks open a new perspective on battery architecture. So far we usually think of the electrolyte shuttling energy back and forth from the electrodes as charging and discharging takes place.
But this concept is so new the possibilities are yet to be discovered. Hopefully we’ll see a lot more on this soon. It will be interesting to see what the advantages and challenges are there to incite more research and development or cast the idea aside. Lets hope there are whole new high level discoveries as this work continues.

