Jun
9
When temperatures fall below freezing, cellphones need to be recharged frequently, and electric cars have shorter driving ranges. This is because their lithium-ion batteries’ anodes get sluggish, holding less charge and drain energy quickly.
The research team reported their findings in ACS Central Science.
Lithium-ion batteries are great for powering rechargeable electronics because they can store a lot of energy and have long lifespans. But when temps fall below freezing, these energy sources’ electrical performance declines, and when conditions are cold enough, they can fail to transfer any charge. It’s why some people living in the U.S. Midwest have trouble with their electric cars in the dead of winter, and why it’s risky to use these batteries in space explorations.
A graphic comparison of energy availability from lithium ion batteries as cold grows deeper using smooth vs bumpy anodes. Image Credit: School of Chemical Engineering and Technology, Tianjin University. Click the research paper link for a larger and more images.
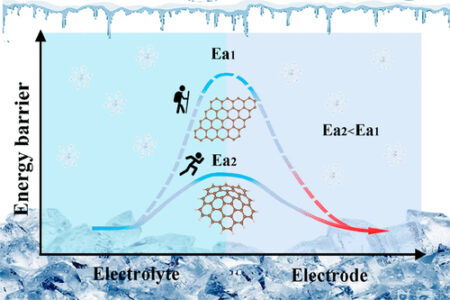
Recently, scientists determined that the flat orientation of graphite in the anode is responsible for the drop in a lithium-ion battery’s energy storage capacity in the cold. So, Xi Wang, Jiannian Yao and colleagues wanted to modify the surface structure of a carbon-based material to improve the anode’s charge transfer process.
To create the new material, the researchers heated a cobalt-containing zeolite imidazolate framework (known as ZIF-67) at high temperatures. The resulting 12-sided carbon nanospheres had bumpy surfaces that demonstrated excellent electrical charge transfer capabilities.
Then the team tested the material’s electrical performance as the anode, with lithium metal as the cathode, inside a coin-shaped battery. The anode demonstrated stable charging and discharging at temperatures from 77 F to -4 F and maintained 85.9% of the room temperature energy storage capacity just below freezing. In comparison, lithium-ion batteries made with other carbon-based anodes, including graphite and carbon nanotubes, held almost no charge at freezing temperatures.
When the researchers dropped the air temperature to -31 F, the anode made with bumpy nanospheres was still rechargeable, and during discharge, released nearly 100% of the charge put into the battery.
Incorporating the bumpy nanosphere material into lithium-ion batteries could open up the possibilities for using these energy sources at extremely low temperatures, the researchers say.
***
This is great news for a lot of the world! For many of us the cell phone in particular has to be up close and real personal when out in the cold. Should the electric vehicle market make progress into colder climates this kind of technology will be crucial to success.
For now the technology is still in its infancy, but there should be more innovation on the way. Lots of battery using technology needs this development to mature and expand out of doors.
Its a “Whew” kind of news, as some of us were wondering if research and product developers were even paying attention.

