Aug
20
Research Reveals In Part Why Perovskite Degrades In The Sun
August 20, 2020 | Leave a Comment
Research by scientists at the Eindhoven University of Technology and universities in China and the US are exploring the causes of perovskite degradation in sunlight. The research may pave the way for designing new perovskite compositions for the ultimate stable solar cells.
Perovskite solar cells are at the center of much recent solar research. The material is cheap and almost as efficient as silicon. However, perovskite cells have a love-hate-relationship with the sun. The light they need to generate electricity, also impairs the quality of the cells, limiting efficiency and stability over time. The new research, published in Joule, now sheds new light on the causes of this degradation.
Perovskite is an attractive alternative to silicon, because it’s abundant and easy to produce. What’s more, over the past decade, the performance of perovskite solar cells has improved dramatically, with efficiency rates reaching more than 25 percent, which is close to the state-of-art for silicon solar cells.
The new research focuses on perovskite solar cells made from formamidinium-cesium lead iodide, a halide compound that has become increasingly popular as it combines high efficiency and reasonable heat resistance with low manufacturing costs.
However, solar panels made of this particular compound have a rather ambivalent relationship with sunlight, a problem that is well-known in the field, but barely understood. While the light of the sun feeds it with the much-wanted energy to convert into electricity, it also impairs the stability of the cells. Over time this affects their performance.
To understand why this is the case, the researchers at TU/e, Peking University and University of California San Diego did both practical experiments – monitoring the photovoltaic performance of the panels over 600 hours of exposure and characterizing the degraded perovskites – and theoretical analysis.
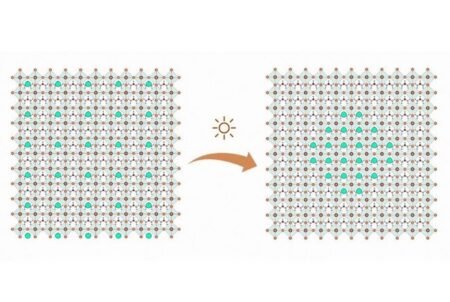
The atomic structure of mixed FACsPbI3 perovskite, where it separates into two CsPbI3 (green region) and FAPbI3 clusters under light excitation. Image Credit: Eindhoven University of Technology. Click image for the largest view.
From this they concluded that sunlight generates charged particles in the perovskite, which tend to flow to places in the solar panel where the band gap (the minimum amount of energy needed for generating the free electrons) is lowest, in this case the formamidinium perovskite. The resulting energy differences make the mixed compounds that worked together so well to make the cell efficient, fall apart into separate clusters. It appears that especially the cesium-heavy clusters (the green dots in the image) are photoinactive and current-blocking, limiting the performance of the device.
According to Shuxia Tao, who together with PhD candidate Zehua Chen and her colleague Geert Brocks was responsible for the TU/e part of the research, the new findings are one step further to finding the way to possible solutions said, “By combining macroscopic tests, microscopic materials characterization and atomistic modeling, we were able to thoroughly understand the instability of halide perovskites that are intrinsic to device operation. This opens the possibility for designing new perovskite compositions for the ultimate stable solar cells.”
Possible strategies include using additives to enhance the chemical interaction inside the materials in the panels, tuning the band gaps by using other elements like bromide and rubidium instead of iodide and cesium, or modifying the energy levels to extract photo-carriers more efficiently.
Tao stressed that more research is needed to see what solution works best. In addition, separation of halide compounds is not the only cause for perovskite degradation. These additional causes require separate analysis.
Everyone note that “Tao stressed that more research is needed”, wise observation as exciting as perovskite seems, it remains far from commercial readiness. This is not to suggest the research has not come very far indeed. One could make a good bet thinking perovskite panels will get to market someday. But it still looks to be a way off.

