Mar
11
Two Major Improvements to Potassium Batteries
March 11, 2020 | Leave a Comment
First to get out a press release was Australian scientists who have developed a nonflammable electrolyte for potassium and potassium-ion batteries. Then a couple weeks later researchers at Rensselaer Polytechnic demonstrated how they can overcome a persistent challenge known as dendrites to create a potassium metal battery that performs nearly as well as a lithium-ion battery. It looks like lithium ion has challenges coming.
The University of Wollongong, Australia researchers wrote that the novel electrolyte based on an organic phosphate makes the batteries safer and also allows for operation at reduced concentrations, which is a necessary condition for large-scale applications. That research has been published in the journal Angewandte Chemie. This team expects potassium to be the next battery generation of energy-storage systems beyond lithium technology.
Lithium-ion technology still dominates energy-storage applications, but it has intrinsic disadvantages, among which are the price, environmental issues, and the flammability of the electrolyte. So, in next-generation technologies, scientists are replacing the lithium ion with more abundant and much cheaper ions, such as the potassium ion. However, potassium and potassium-ion batteries also face safety issues, and nonflammable electrolytes are not, so far, yet available for them.
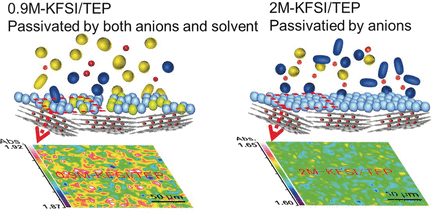
Materials scientist Zaiping Guo, and her team from the University of Wollongong, Australia have found a solution. The researchers developed an electrolyte based on a flame-retardant material and adapted it for use in potassium batteries. Besides providing nonflammability, it could be operated in batteries at concentrations that are suitable for large-scale applications, wrote the scientists.
This novel electrolyte contained triethyl phosphate as the sole component of the solvent. This substance is known as a flame retardant. It has been tested in lithium-ion batteries, but only very high concentrations provided enough stability for long-term operation, too high for industrial applications. The battery industry demands dilute electrolytes, which are cheaper and ensure better performance.
By using potassium ions, however, the concentrations could be reduced, the authors reported. They combined the phosphate solvent with a commonly available potassium salt and obtained an electrolyte that did not burn and allowed stable cycling of the assembled battery concentrations of 0.9 to 2 moles per liter, which are concentrations that are suitable for larger scales; for example, in smart-grid applications.
Key to that performance was the formation of a uniform and stable solid-electrolyte interphase layer, according to the authors. They observed this layer, which ensures operability of the electrodes, only with the phosphate electrolyte. Conventional carbonate-based electrolytes were unable to build up this layer. The authors also reported high cycling stability; whereas, under the same conditions, the conventional carbonate-based electrolyte decomposed.
Guo and her team have demonstrated that next-generation potassium-ion batteries can be made safe by using a novel inorganic, phosphate-based electrolyte. They suggest that electrolytes based on flame retardants can be developed further and could be used for the design of other nonflammable battery systems.
Almost meanwhile, the Rensselaer team demonstrated how they can overcome a persistent challenge known as dendrites to create a metal battery that performs nearly as well as a lithium-ion battery, but relies on potassium – a much more abundant and less expensive element.
Batteries contain two electrodes — a cathode on one end and an anode on the other. If you were to look inside a lithium-ion battery you’d typically find a cathode made of lithium cobalt oxide and an anode made of graphite. During charging and discharging, lithium ions flow back and forth between these two electrodes.
In this setup, if researchers were to simply replace lithium cobalt oxide with potassium cobalt oxide, performance would drop. Potassium is a larger and heavier element and, therefore, less energy dense. Instead, the Rensselaer team looked to boost potassium’s performance by also replacing the graphite anode with potassium metal.
Nikhil Koratkar, an endowed professor of mechanical, aerospace, and nuclear engineering at Rensselaer and the lead author on this paper said, “In terms of performance, this could rival a traditional lithium-ion battery.”
While metal batteries have shown great promise, they have also traditionally been plagued by accumulation of metal deposits, called dendrites, on the anode. Dendrites are formed because of non-uniform deposition of potassium metal as the battery undergoes repeated cycles of charging and discharging. Over time, Koratkar explained, the conglomerates of potassium metal become long and almost branch-like.
If they grow too long, they will eventually pierce the insulating membrane separator meant to keep the electrodes from touching each other and shorting out the battery. Heat is created when a battery shorts and has the potential to set the organic electrolyte within the device on fire.

Effect of thermal annealing on dendrites of potassium metal electrodes. SEM images after (a) cycling at ~0.5 mA cm-2for 50 cycles and after thermal annealing of the cycled cell at ~40 ̊C for (b) ~36 hours and (c) ~72 hours. Image Credit: Rensselaer Polytechnic Institute. Click image for the largest view.
In their paper, Koratkar and his team, which included Prateek Hundekar, a doctoral student at Rensselaer, and researchers from the University of Maryland, including Chunsheng Wang, a professor of chemical and biomolecular engineering, explained how their solution to that problem paves the way for practical consumer use. By operating the battery at a relatively high charge and discharge rate, they can raise temperature inside the battery in a well-controlled manner and encourage the dendrites to self-heal off the anode.
Koratkar compared the self-healing process to what happens to a pile of snow after a storm has ended. The wind and the sun help move the flakes off the mound of snow, shrinking its size and eventually flattening it out.
In a similar way, while the temperature increase within the battery won’t melt the potassium metal, it does help to activate surface diffusion so the potassium atoms move laterally off the “pile” they’ve created, effectively smoothing the dendrite out.
“With this approach, the idea is that at night or whenever you’re not using the battery, you would have a battery management system that would apply this local heat that would cause the dendrites to self-heal,” Koratkar said.
Koratkar and his team previously demonstrated a similar method of self-healing with lithium metal batteries, but they found the potassium metal battery required much less heat to complete the self-healing process. That promising finding, Koratkar said, means a potassium metal battery could be more efficient, safe, and practical.
“I want to see a paradigm shift to metal batteries,” Koratkar said. “Metal batteries are the most efficient way to construct a battery; however, because of this dendrite problem they have not been feasible. With potassium, I’m more hopeful.”
It does look like potassium chemistry has the potential to get to market. The low cost and hopefully lower risks in operation has to offer some large installation customers higher and better incentives to engage.
Time will tell, but lithium just quietly gets more and more expensive. For consumers its better to have the lower cost alternative ready than go through product cycles with high prices for the energy storage.