Oct
9
New Battery Chemistry of Aluminum Doubles Energy Density
October 9, 2019 | 1 Comment
Chalmers University of Technology and the National Institute of Chemistry, Slovenia have new concept for an aluminum battery has twice the energy density as previous versions, is made of abundant materials, and could lead to reduced production costs and environmental impact. The press release offers the idea that it has potential for large scale applications, including storage of solar and wind energy.
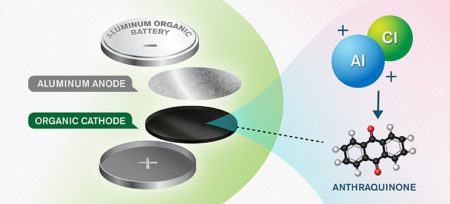
The new concept consists of an anode and cathode made of aluminum and an anthraquinone-based organic material, respectively. The organic cathode material enables efficient storage of the positive charge carriers from an aluminum and chlorine-based electrolyte – the solution in which ions can move between the electrodes. Image Credit: Illustrator Yen Strandqvist. Chalmers University. Click image for the largest view.
Using aluminum battery technology could offer several advantages, including a high theoretical energy density, and the fact that there already exists an established industry for its manufacturing and recycling. Compared with today’s lithium-ion batteries, the researchers’ new concept could result in markedly lower production costs.
The team’s research paper has been published in the journal Energy Storage Materials.
Patrik Johansson, Professor at the Department of Physics at Chalmers explains, “The material costs and environmental impacts that we envisage from our new concept are much lower than what we see today, making them feasible for large scale usage, such as solar cell parks, or storage of wind energy, for example.”
“Additionally, our new battery concept has twice the energy density compared with the aluminum batteries that are ‘state of the art’ today,” he added.
Previous designs for aluminum batteries have used the aluminum as the anode (the negative electrode) and graphite as the cathode (the positive electrode). But graphite provides too low an energy content to create battery cells with enough performance to be useful.
But in the new concept, presented by Patrik Johansson and Chalmers, together with a research group in Ljubljana led by Robert Dominko, the graphite has been replaced by an organic, nanostructured cathode, made of the carbon-based molecule anthraquinone.
The anthraquinone cathode has been extensively developed by Jan Bitenc, previously a guest researcher at Chalmers from the group at the National Institute of Chemistry in Slovenia.
The advantage of this organic molecule in the cathode material is that it enables storage of positive charge-carriers from the electrolyte, the solution in which ions move between the electrodes, which make possible higher energy density in the battery.
Chalmers researcher Niklas Lindahl, who studies the internal mechanisms which govern energy storage explained, “Because the new cathode material makes it possible to use a more appropriate charge-carrier, the batteries can make better usage of aluminum’s potential. Now, we are continuing the work by looking for an even better electrolyte. The current version contains chlorine – we want to get rid of that.”
So far, there are no commercially available aluminum batteries, and even in the research world they are relatively new. The question is if aluminum batteries could eventually replace lithium-ion batteries.
Professor Johansson continued, “Of course, we hope that they can. But above all, they can be complementary, ensuring that lithium-ion batteries are only used where strictly necessary. So far, aluminum batteries are only half as energy dense as lithium-ion batteries, but our long-term goal is to achieve the same energy density. There remains work to do with the electrolyte, and with developing better charging mechanisms, but aluminum is in principle a significantly better charge carrier than lithium, since it is multivalent, which means every ion ‘compensates’ for several electrons. Furthermore, the batteries have the potential to be significantly less environmentally harmful.”
Your humble writer has a question, too. Perhaps the technology is already close to moving into the lead acid battery market? Its huge as well, and could stand some more power in a better battery that’s cheaper as well.
Comments
1 Comment so far


I have known people who worked at EXCIDE making lead acid batteries. They all suffered from lead poisoning to some extent. Even those who always wore the safety equipment.
This would be huge for their life and health.