Mar
14
Fuel Cell With 10 Times the Power With 10% of the Platinum
March 14, 2019 | 2 Comments
Electric vehicles running on fuel cells tout zero emissions and higher efficiency, but expensive platinum is holding them back from entering a larger market.
The 2019 Toyota Mirai electric vehicle touts zero emissions, thanks to a fuel cell that runs on hydrogen instead of gasoline. But the Mirai has barely left California, partly because today’s fuel cell electrodes are made of super expensive platinum.
Cutting down on the platinum would also cut costs, allowing more electric cars to go to market.
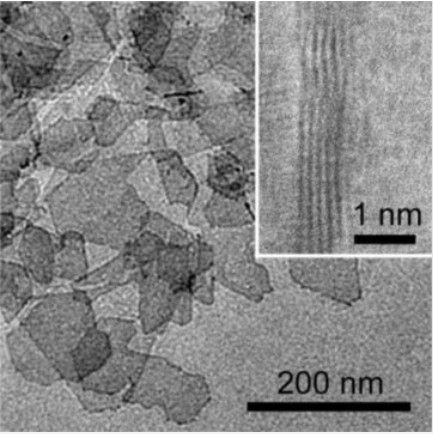
A platinum-like metal only five atomic layers thick is “just right” for optimizing the performance of a fuel cell electrode. Image Credit: Johns Hopkins University. Image by Lei Wang. Click image for the largest view.
The new method borrows some thinking from “Goldilocks” – just the right amount – for evaluating how much metal would be required for fuel cell electrodes. The technique uses the forces on a metal’s surface to identify the ideal electrode thickness.
Jeffrey Greeley, professor of chemical engineering at Purdue said, “There is exactly the right amount of metal that will give fuel cell electrodes the best properties. If they are too thick or too thin, the main reaction for deploying a fuel cell doesn’t work as well, so there’s sort of a Goldilocks principle here.”
The researchers tested their theory on palladium, a metal very similar to platinum.
“We’re essentially using force to tune the properties of thin metal sheets that make up electrocatalysts, which are part of the electrodes of fuel cells,” Greeley said. “The ultimate goal is to test this method on a variety of metals.”
Fuel cells convert hydrogen, combining with oxygen, into electricity through a so-called oxygen-reduction reaction that an electrocatalyst starts. Finding exactly the right thickness stresses the surface of the electrocatalyst and enhances how well it performs this reaction.
Researchers in the past have tried using outside forces to expand or compress an electrocatalyst’s surface, but doing so risked making the electrocatalyst less stable.
Instead, Greeley’s group predicted through computer simulations that the inherent force on the surface of a palladium electrocatalyst could be manipulated for the best possible properties.
According to the simulations, an electrocatalyst five layers thick, each layer as thin as an atom, would be enough to optimize performance.
Zhenhua Zheng, a Purdue postdoctoral researcher in chemical engineering, and co-first and co-corresponding author on this paper said, “Don’t fight forces, use them. This is kind of like how some structures in architecture don’t need external beams or columns because tensional and compressive forces are distributed and balanced.”
Experiments in Chao Wang’s lab at Johns Hopkins confirmed the simulation predictions, finding that the method can increase catalyst activity by 10 to 50 times, using 90 percent less of the metal than what is currently used in fuel cell electrodes.
This is because the surface force on the atomically thin electrodes tunes the strain, or distance between atoms, of the metal sheets, altering their catalytic properties.
“By tuning the material’s thickness, we were able to create more strain. This means you have more freedom to accelerate the reaction you want on the material’s surface,” Wang said.
The study was supported by multiple entities, including the U.S. Department of Energy, National Energy Research Scientific Computing Center and the National Science Foundation.
The work aligns with Purdue’s Giant Leaps celebration, acknowledging the university’s global advancements made toward a sustainable economy and planet as part of Purdue’s 150th anniversary. This is one of the four themes of the yearlong celebration’s Ideas Festival, designed to showcase Purdue as an intellectual center solving real-world issues.
The hydrogen economy folks might well be breaking out the Champaign. It sounds like the team is going to try other catalysts as well. The fuel cell might be getting its marketable legs at last. Now, to find the safe cheap way to store hydrogen without it getting away.
Comments
2 Comments so far


Hydrogen is a bad fuel to handle. Fuel cells using ethanol, methanol or liquid fuels should be preferred.
New energy contributes a lot to the world