Sep
5
Single Atom Catalysts for Fuel Cells
September 5, 2018 | Leave a Comment
Washington State University (WSU) researchers have developed a new way to make low-cost, single-atom catalysts for fuel cells. The advance that could make important clean energy technology more economically viable.
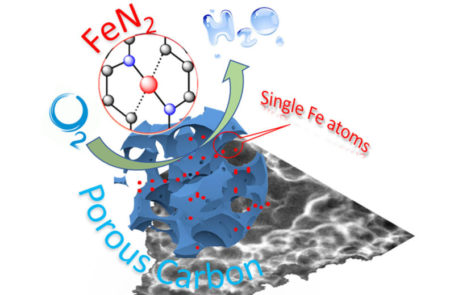
Schematic illustration of single-atom catalyst anchored on porous carbon. Image Credit: Washington State University. Click image for the largest view.
The team’s study results have been published in the journal Advanced Energy Materials.
Hydrogen fuel cells will be critical for a hydrogen fueled energy economy as they are well more than two times as efficient at creating electricity than polluting combustion engines. The effluent product or waste material is water vapor.
Currently, the high price of the platinum-based catalysts that are used for the chemical reaction in fuel cells significantly hinders their commercialization.
Instead of the rare and expensive platinum, researchers would like to use non-precious metals, such as iron or cobalt. But reactions with these abundantly available metals tend to stop working after a short time.
Qiurong Shi, postdoctoral researcher in the School of Mechanical and Materials Engineering (MME) and a co-first author on the paper said, “Low-cost catalysts with high activity and stability are critical for the commercialization of the fuel cells.”
The researchers technological breakout is developing single-atom catalysts that work as well in the laboratory setting as using precious metals. The researchers have been able to improve the stability and activity of the non-precious metals by working with them at the nanoscale as single-atom catalysts.
WSU MME professor Yuehe Lin led the team in this new work. They used iron or cobalt salts and the small molecule glucosamine as precursors in a straightforward high temperature process to create the single-atom catalysts. The process can significantly lower the cost of the catalysts and could be easily scaled up for production.
The iron-carbon catalysts they developed were more stable than commercial platinum catalysts. They also maintained good activity and didn’t become contaminated, which is often a problem with common metals.
Chengzhou Zhu, a first author on the paper who developed the high temperature process said, “This process has many advantages. It makes large-scale production feasible, and it allows us to increase the number and boost the reactivity of active sites on the catalyst.”
Lin’s group collaborated on the project with Scott Beckman, an MME associate professor at WSU, as well as with researchers at Advanced Photon Source at Argonne National Laboratory and Brookhaven National Laboratory for materials characterization.
“The advanced materials characterization user facility at the national laboratories revealed the single-atom sites and active moieties of the catalysts, which led to the better design of the catalysts,” said Lin.
The fuel cell is for many the answer to high power, portability and mobility, and efficiency. When such devices exist in economically operating numbers the demand for hydrogen and di-hydrogen gases will take off. Then the hydrogen storage issue will come up in a big way.
There looks like many hydrogen production ideas are viable. But to really find out who has what market power the fuel cells have to drive some demand. We’re getting there.

