May
3
University of Exeter has pioneered a new technique to produce hydrogen from sunlight using a new photo-electrode. The photo-electrode is an electrode that absorbs light before initializing electrochemical transformations to extract the hydrogen from water. The newly invented photo-electrode is made from nanoparticles of the elements lanthanum, iron and oxygen.
The researchers believe this new type of photo-electrode is not only cheap to produce, but can also be recreated on a larger scale for mass and worldwide use of water splitting.
In this new research, the team utilized lanthanum iron oxide to create a semi-conducting material that gave the ideal results for the production of hydrogen from water using sunlight, making it the strongest candidate yet for renewable hydrogen generation.
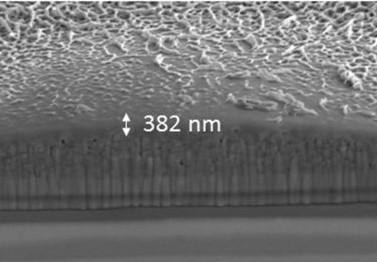
FIB-SEM cross section of LaFeO3 photo-electrode. Image Credit: Govinder Pawar, University of Exeter . Click image for the largest view.
Govinder Pawar, lead author on the paper and based at the University of Exeter’s Environment and Sustainability Institute on the Penryn Campus in Cornwall said, “We have shown that our LaFeO3 photo-electrode has ideal band alignments needed to split water into its constituents (H2 and O2) spontaneously, without the need of an external bias. Moreover, our material has excellent stability where after 21 hours of testing it does not degrade, ideal for water splitting purpose. We are currently working on further improving our material to make it more efficient to produce more hydrogen.”
Photo electro chemical (PEC) water splitting to produce solar fuel (hydrogen) has long been considered as the Holy Grail to a carbon-free hydrogen economy. The PEC concept to produce solar fuel is to emulate the natural photosynthesis using man made materials.
The bottle-neck in realizing the concept practically has been the difficulty in identifying stable low-cost semiconductors that meet the thermodynamic and kinetic criteria for photoelectrolysis. The team fabricated the new p-type LaFeO3 photo-electrode using an inexpensive and scalable spray pyrolysis method.
The team’s nanostructured LaFeO3 photo-electrode resulted in spontaneous hydrogen evolution from water without any external bias applied. Moreover, the photo-electrode has a faradaic efficiency of 30% and showed excellent stability over 21 hours.
To the best of the team’s knowledge this is the first time hydrogen has been produced spontaneously during a water splitting test without any external bias being applied using LaFeO3 photo-electrode as a single material. These findings demonstrate that LaFeO3 is a potential candidate to act as a photo-electrode for unassisted PEC water splitting to generate solar fuel (hydrogen) cost effectively.
This might be the breakthrough the hydrogen folks have been looking for. The team isn’t introducing an outside power source nor separately generating current for electrolysis.
While this is a report on the first trial in the lab and there are issues to be solved, there is a very encouraging foothold in a new developing field. The team is likely hard at improving it at this moment.
Your humble writer suspects we will look back at this and note “this was the breakthrough.”
Congratulations are in order for the Exeter team. Well done indeed!
Comments
2 Comments so far


I disagree with your statement: “making it the strongest candidate yet for renewable hydrogen generation.”
Hypersolar http://www.hypersolar.com has been developing a nano-particle water splitter for years and is almost ready for a pilot plant.
They are indeed the closest to commercialization.
Please see:
https://finance.yahoo.com/news/hypersolar-announces-record-294-hours-073000272.html
HyperSolar announces record hydrogen production