Mar
15
Breakout For the Solid State Lithium Ion Battery
March 15, 2018 | 1 Comment
Shinshu University researchers have developed a new way to improve solid state lithium ion battery efficiency. Through the growth of a cubic crystal layer, the scientists have created a thin and dense connecting layer between the electrodes of the battery.
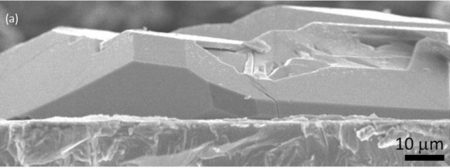
Cross-sectional SEM image of a ceramic separator in a solid state lithium ion battery. Image Credit: Center for Energy and Environmental Science, Department of Materials Chemistry, Shinshu University. Click image for the largest view.
Rechargeable lithium ion batteries power such devices as cell phones, laptops, power tools, and are even hoped to store power for the electrical grid. But they’re particularly sensitive to temperature fluxes, and have been known to cause fires or even explosions. In response to the problems with liquid electrolytes, scientists are working toward developing a better all-solid-state battery without liquid.
Professor Nobuyuki Zettsu from the Center for Energy and Environmental Science in the Department of Materials Chemistry of Shinshu University in Japan and the director of the center, Professor Katsuya Teshima, led the research for a liquid electrolyte replacement.
The authors research results have been published in Scientific Reports.
Nobuyuki Zettsu, the first author on the paper explained the background with, “Owing to some intrinsic characteristics of liquid electrolytes, such as low lithium transport number, complex reaction at the solid/liquid interface, and thermal instability, it has not been possible to simultaneously achieve high energy and power in any of the current electrochemical devices.”
Zettsu continued, “Despite the expected advantages of all-solid-state batteries, their power characteristic and energy densities must be improved to allow their application in such technologies as long-range electric vehicles. The low rate capabilities and low energy densities of the all-solid-state batteries are partly due to a lack of suitable solid-solid heterogeneous interface formation technologies that exhibit high iconic conductivity comparable to liquid electrolyte systems.”
Zettsu and his team grew garnet-type oxide solid electrolyte crystals in molten LiOH used as a solvent (flux) on a substrate that bonded the electrode into a solid state as they grew. A specific crystal compound known to grow cubically allowed the researchers to control the thickness and connection area within the layer, which acts as a ceramic separator.
“Electron microscopy observations revealed that the surface is densely covered with well-defined polyhedral crystals. Each crystal is connected to neighboring ones,” wrote Zettsu, adding that the newly grown crystal layer could be the ideal ceramic separator when stacking the electrolyte layer on the electrode layer.
“We believe that our approach having robustness against side reactions at the interface could possibly lead to the production of ideal ceramic separators with a thin and dense interface,” wrote Zettsu, noting that the ceramics used in this particular experiment were too thick to be used in solid batteries. “However, as long as the electrode layer can be made as thin as 100 microns, the stacking layer will operate as a solid battery.”
One hundred microns is about the width of a human hair, and slightly less than twice the thickness of a standard electrode layer in contemporary lithium-ion batteries.
“All-solid-state batteries are promising candidates for energy storage devices,” Zettsu said, noting that several collaborations between researchers and private companies are already underway with the ultimate goal of displaying all-solid-state battery samples at the 2020 Olympic games in Tokyo.
Zettsu and other researchers plan to fabricate prototype cells for electric vehicle use and for wearable devices by 2022.
Other collaborators on this project include researchers from the Institute for Materials Research at Tohoku University, Frontier Research Institute for Materials Science at Nagoya Institute of Technology, and the National Institute for Materials Science.
This is welcome news indeed, especially for those using lithium batteries in sub-freezing temperatures where the available power is greatly diminished. Thus, your humble writer hopes this team picks up the pace!
Battery chemical activity is getting more and more thoroughly understood and material research is gaining new insights. Research like this sure brightens up the future.
Comments
1 Comment so far


What company is manufacturing this battery