Jan
4
A New Model For Energy Storage
January 4, 2018 | 7 Comments
A research team from the International Institute for Carbon-Neutral Energy Research at Kyushu University has built a flow-type polymer electrolyte cell for power storage, a device to store energy in chemical form through continuous electrolysis.
This already competitive energy-storage device could be used to balance out the fluctuations in renewable power supplies. Storing electricity is not without its challenges. Interest in renewable energy continues to grow, but many renewables can be frustratingly intermittent, when the sun stops shining, or the wind stops blowing, the power stops. The only credible answer is a fluctuating supply that can be partly smoothed-out by energy storage during peak production times.
The new flow-type polymer electrolyte cell reduces oxalic acid (OX) to glycolic acid, which has a higher volumetric energy-storage capacity than hydrogen gas. A newly fabricated TiO2 cathode enhanced the speed and efficiency of OX reduction.
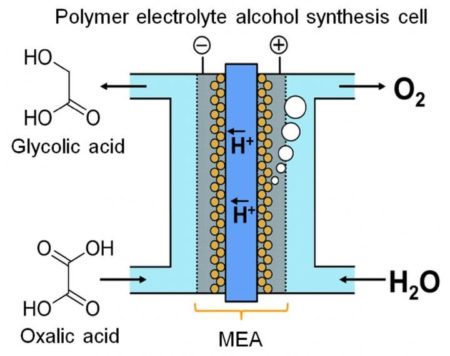
A Kyushu University research team realized continuous electrochemical synthesis of an alcohol compound from a carboxylic acid using a polymer electrolyte alcohol electrosynthesis cell, which enables direct power charge into alcoholic compound. Image Credit: Masaaki Sadakiyo, International Institute for Carbon-Neutral Energy Research, Kyushu University. Click image for the largest view.
The researchers noted that glycolic acid (GC) has a much greater energy capacity than hydrogen, one of the more popular energy-storage chemicals. GC can be produced by four-electron reduction of oxalic acid (OX), a widely available carboxylic acid. The team devised an electrolytic cell based on a novel membrane-electrode assembly. Sandwiched between two electrodes are an iridium oxide-based anode and a titanium dioxide (TiO2)-coated titanium (Ti) cathode, linked by a polymer membrane.
Study lead author Masaaki Sadakiyo explained, “Flow-type systems are very important for energy storage with liquid-phase reaction. Most electrolyzers producing alcohols operate a batch process, which is not suitable for this purpose. In our device, by using a solid polymer electrolyte in direct contact with the electrodes, we can run the reaction as a continuous flow without addition of impurities (e.g. electrolytes). The OX solution can effectively be thought of as a flowable electron pool.”
Another key consideration is the cathode design. The cathodic reaction is catalyzed by anatase TiO2. To ensure a solid connection between catalyst and cathode, the team “grew” TiO2 directly on Ti in the form of a mesh or felt. Electron microscope images show the TiO2 as a wispy fuzz, clinging to the outside of the Ti rods like a coating of fresh snow. In fact, its job is to catalyze the electro-reduction of OX to GC. Meanwhile, at the anode, water is oxidized to oxygen.
The team found that the reaction accelerated at higher temperatures. However, turning the heat up too high encouraged an unwanted by-process – the conversion of water to hydrogen. The ideal balance between these two effects was at 60°C (156.2º F). At this temperature, the device could be further optimized by slowing the flow of reactants, while increasing the amount of surface area available for the reaction.
Interestingly, even the texture of the fuzzy TiO2 catalyst made a major difference. When TiO2 was prepared as a “felt,” by growing it on thinner and more densely packed Ti rods, the reaction occurred faster than on the “mesh” – probably because of the greater surface area. The felt also discouraged hydrogen production, by blanketing the Ti surface more snugly than the mesh, preventing the exposure of bare Ti.
Co-author Miho Yamauchi said, “In the right conditions, our cell converts nearly 100% of OX, which we find very encouraging. We calculate that the maximum volumetric energy capacity of the GC solution is around 50 times that of hydrogen gas. To be clear, the energy efficiency, as opposed to capacity, still lags behind other technologies. However, this is a promising first step to a new method for storing excess current.”
This is an amazing discovery or invention. However it is described, this new technology is a welcome birth. There doesn’t seem to be any dreadfully expensive ingredients, nor wildly exotic process technologies in construction. It works best at sub fresh made coffee temperatures.
Yes it is the first step, newly born. But the potential here is fantastic, a word seldom used here, so lets hope the innovators, developers and engineers get enthused. Up to 50 times the energy capacity of hydrogen in an unpressurized container of glycolic acid, an ingredient in skin care. Its a tremendous incentive!
Comments
7 Comments so far


Up to 50 times the energy capacity of hydrogen in an unpressurized container of glycolic acid, an ingredient in skin care. Its a tremendous incentive!
Yes it is the first step, newly born. But the potential here is fantastic, a word seldom used here, so lets hope the innovators, developers and engineers get enthused.
The team devised an electrolytic cell based on a novel membrane-electrode assembly. Sandwiched between two electrodes are an iridium oxide-based anode and a titanium dioxide (TiO2)-coated titanium (Ti) cathode, linked by a polymer membrane.
The felt also discouraged hydrogen production, by blanketing the Ti surface more snugly than the mesh, preventing the exposure of bare Ti.
In the right conditions, our cell converts nearly 100% of OX, which we find very encouraging. We calculate that the maximum volumetric energy capacity of the GC solution is around 50 times that of hydrogen gas.
how can we sponsor this research?
Contact them and ask.