Nov
1
New Catalysts To Split Water For Hydrogen Fuel
November 1, 2017 | 4 Comments
Institute of Chemical Research of Catalonia (ICIQ) and URV researchers have designed a new catalyst that reduces the cost of electrolytic hydrogen production. The new catalysts reduce the amount of electricity needed to break the chemical bonds, speed up the reaction and minimize the energy waste.
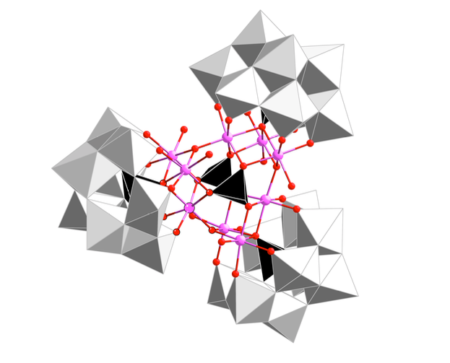
The new cobalt-tungsten polyoxometalate molecule. Image Credit: Institute of Chemical Research of Catalonia. Click image for the largest view.
Galán-Mascarós explained, “Normally, hydrogen is obtained from using a cheap process called steam reforming. But this is not clean hydrogen, this process uses natural gas and produces carbon dioxide and other contaminants. Breaking the water molecule is cleaner, but it’s not easy. We need to develop new cheap, efficient catalysts that allow us to obtain hydrogen at a competitive price.” To date, the best catalysts are based in iridium oxides, but iridium is a very expensive and scarce precious metal.
The chemists at ICIQ and URV discovered a compound made of cobalt and tungsten -technically called a polyoxometalate – which can catalyze water splitting better than iridium.
Marta Blasco-Ahicart, postdoctoral researcher at ICIQ and first author of the Nature Chemistry paper explained, “Polyoxometalates are nanometric molecular oxides that combine the best of two worlds, the great activity of oxides and the versatility of molecules. Our polyoxometalates are way cheaper than iridium and allow us to work in acidic media, the optimal media to generate oxygen that is normally a drawback for catalysts, which are usually consumed by the acid.”
Joaquín Soriano, co-author of the paper and currently a postdoctoral researcher at Trinity College in Dublin said, “Our catalysts work specially well when we work with low voltages. That may seem an issue, but is rather an advantage, it saves electricity and will allow us, soon, to obtain the energy required for water splitting from renewable sources like solar panels.”
Moreover, researchers present in their paper an additional discovery. Supporting the catalysts in a partially hydrophobic -water repellent- material, the efficiency of the process improves. This generates a ‘waterproof’ reactor’ where electrolysis advances quicker, and also enhances the lifetime of catalysts.
The new methodology not only improves the performance of the new cobalt-tungsten polyoxometalates, but also with a lot of different catalytic systems. Nowadays, researchers are investigating new ways of taking advantage of this new finding, developing new hydrophobic scaffolds to further boost the efficiency of water splitting, a fundamental step towards the evolution of artificial photosynthesis.
Freeing up hydrogen gets easier and better all the time. But the storage bugaboo is still out there and remains the eight hundred pound gorilla problem.
Comments
4 Comments so far


The new methodology not only improves the performance of the new cobalt-tungsten polyoxometalates, but also with a lot of different catalytic systems.
Perhaps all of us will benefit from a lot from the methodology. My dear friend, thanks for your sharing.
Nowadays, researchers are investigating new ways of taking advantage of this new finding, developing new hydrophobic scaffolds to further boost the efficiency of water splitting, a fundamental step towards the evolution of artificial photosynthesis.
Freeing up hydrogen gets easier and better all the time. But the storage bugaboo is still out there and remains the eight hundred pound gorilla problem.