Mar
26
Graphene May Make the Super Battery a Reality
March 26, 2013 | 3 Comments
This qualifies as a breakthrough for the cathode half-cell and should accelerate the development of high-power lithium-ion batteries suitable for electric cars and other demanding applications. Vanadium oxide and graphene, separately, are well-studied materials and can be a superior cathode for batteries that could supply both high energy density and significant power density.
Ajayan’s research appears online this month in the American Chemical Society’s journal Nano Letters. The ribbons are thousands of times thinner than a sheet of paper and have a potential that far outweighs current materials for their ability to charge and discharge very quickly. Cathodes built into half-cells for testing at Rice fully charged and discharged in only 20 seconds, an astonishing rate and retaining more than 90% of their initial capacity after more than 1,000 cycles is far better than what consumers experience with cell phones and power tools now.
Ajayan said, “This is the direction battery research is going, not only for something with high energy density but also high power density. It’s somewhere between a battery and a supercapacitor.” Perhaps the term coined by author Phillip J. Farmer decades ago, “the batacitor” is coming into to being.
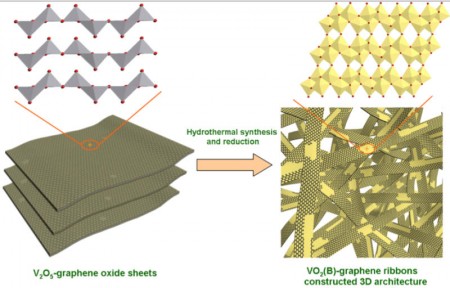
The ribbons also have the advantage of using relatively abundant and cheap materials. “This is done through a very simple hydrothermal process, and I think it would be easily scalable to large quantities,” Ajayan said.
Vanadium oxide has long been considered a material with great potential, and in fact vanadium pentoxide has been used in lithium-ion batteries for its special structure and high capacity. But oxide forms are slow to charge and discharge, due to their low electrical conductivity. The high-conductivity graphene lattice that is literally baked in solves that problem nicely, Ajayan explained, by serving as a speedy conduit for electrons and channels for ions.
The atom-thin graphene sheets bound to the crystals take up very little bulk. In the best samples made at Rice, fully 84% of the cathode’s weight was the lithium absorbing VO2, which held 204 milliamp hours of energy per gram. The researchers, led by Rice graduate student Yongji Gong and lead author Shubin Yang, said they believe that to be among the best overall performance ever seen for lithium-ion battery electrodes.
Yang takes up the explanation saying, “One challenge to production was controlling the conditions for the co-synthesis of VO2 ribbons with graphene.” The process involved suspending graphene oxide nanosheets with powdered vanadium pentoxide (layered vanadium oxide, with two atoms of vanadium and five of oxygen) in water and heating it in an autoclave for hours. The vanadium pentoxide was completely reduced to VO2, which crystallized into ribbons, while the graphene oxide was reduced to graphene. The ribbons, with a web-like coating of graphene, were only about 10 nanometers thick, up to 600 nanometers wide and tens of micrometers in length.
“These ribbons were the building blocks of the three-dimensional architecture,” Yang said. “This unique structure was favorable for the ultrafast diffusion of both lithium ions and electrons during charge and discharge processes. It was the key to the achievement of excellent electrochemical performance.”
Yang and Gong tested the new material and found its capacity for lithium storage remained stable after 200 cycles even at high temperatures as high as 167º F, a temperature that other cathodes commonly decay, even at low charge-discharge rates.
“We think this is real progress in the development of cathode materials for high-power lithium-ion batteries,” Ajayan said. He is also able to suggest the ribbons’ ability to be dispersed in a solvent might make them suitable as a component in the “paintable” batteries developed in his lab.
These are great results born of a high level of innovation.. While lab results for now, its likely replication will take place and more innovation will find the paring to anodes for a very impressive jump in lithium-ion battery value. The value is the curious thing, as from what we’re seen over the past months, the raw materials are not exotic, costly or rare. The processing is new, but the demand paired to disciplined mass production would pull costs down very far, very quickly.
Congratulations to the Rice team, this is impressive work.
Comments
3 Comments so far



Thanks a bunch for sharing this with all folks you really realize what
you’re talking about! Bookmarked.
This is very interesting, You are a very skilled blogger.
I’ve joined your rss feed and look forward to seeking more of your fantastic post. Also, I have shared your website in my social networks!
Whats up. Very cool site!! Guy .. Beautiful .. Superb .. I’ll bookmark your blog and take the feeds additionally…I am glad to find a lot of helpful info right here within the post. Thanks for sharing..