Feb
19
Progress For Drop In Bio Fuel Gasoline Replacements
February 19, 2013 | 2 Comments
Renewable alternatives to gasoline like heavy alcohols such as isobutanol are promising candidates that are getting closer to your gas tank.
The heavier alcohols have more carbon atoms than ethanol with two and methanol with just one. They contain more energy than ethanol, but they are also more compatible with the existing gasoline-based infrastructure. The next two are propanol or isopropyl, the common rubbing alcohol with three carbon atoms and butanol with four carbon atoms. The leading form of butanol is the molecule isobutanol.
If isobutanol is to gain market share scientists need a way to reliably produce huge quantities of it from renewable sources. Therein lies the problem, making larger molecules is much more difficult.
MIT chemical engineers and biologists have devised a new way to dramatically boost isobutanol production in yeast, which naturally make it in small amounts. They engineered yeast so that isobutanol synthesis takes place entirely within the yeast’s mitochondria cell structure. The mitochondria generate energy and also host many biosynthetic pathways. Using this approach, they were able to boost isobutanol production by about 260 percent.
This is still far from the scale needed for industrial production as seen in sugar cane and corn ethanol production. But the advance suggests that this is a promising approach to engineering not only isobutanol but other useful chemicals as well.
Gregory Stephanopoulos, an MIT professor of chemical engineering and one of the senior authors of a paper describing the work in the Feb. 17 online edition of Nature Biotechnology said, “It’s not specific to isobutanol. It’s opening up the opportunity to make a lot of biochemicals inside an organelle that may be much better suited for this purpose compared to the cytosol of the yeast cells.”
Stephanopoulos collaborated with Gerald Fink, an MIT professor of biology and member of the Whitehead Institute, on this research. The lead author of the paper is Jose Avalos, a postdoc at the Whitehead Institute and MIT.
Avalos explains that historically researchers have tried to decrease isobutanol production in yeast, because it can ruin the flavor of wine and beer. However, “now there’s been a push to try to make it for fuel and other chemical purposes,” he said.
Yeast typically produces isobutanol in a series of reactions that take place in two different cell locations. The synthesis begins with pyruvate, a plentiful molecule generated by the breakdown of the sugars such as glucose. Then the pyruvate is transported into the mitochondria, where it can enter many different metabolic pathways, including one that results in production of valine, an amino acid. Alpha-ketoisovalerate (alpha-KIV), a precursor in the valine and isobutanol biosynthetic pathways, is made in the mitochondria in the first phase of isobutanol production.
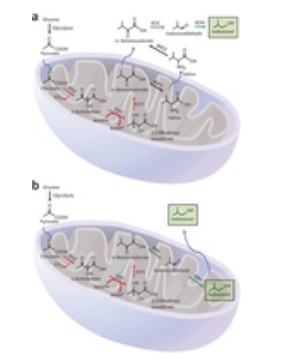
Butanol Yeast Pathway Illustration. Click link to the Nature Biotechnology page for more information.
Valine and alpha-KIV can be transported out to the cytoplasm, where they are converted by a set of enzymes into isobutanol. Other researchers have tried to express all the enzymes needed for isobutanol biosynthesis in the cytoplasm. But, it’s difficult to get some of those enzymes to function in the cytoplasm as well as they do in the mitochondria.
The MIT researchers took the opposite approach and pushed back the yeast’s internal process: They moved the second phase that naturally occurs in the cytoplasm back into the mitochondria. They achieved this by engineering the metabolic pathway’s enzymes to express a tag normally found on a mitochondrial protein, directing the cell to send them into the mitochondria.
This enzyme relocation boosted the production of isobutanol by 260 percent, and yields of two related alcohols, isopentanol and 2-methyl-1-butanol, went up even more — 370 and 500 percent, respectively.
That’s pretty good news. Pentanol is a five-carbon atom alcohol!
The researchers say there are likely several explanations for the dramatic increase. Avalos said one strong possibility, which remains difficult to prove experimentally, is that clustering the enzymes together makes it more likely that the reactions will occur.
Another possible explanation is that moving the second half of the pathway into the mitochondria makes it easier for the enzymes to snatch up the limited supply of precursors before they can enter another metabolic pathway.
Avalos’ thinks perhaps, “Enzymes from the second phase, which are naturally out here in the cytoplasm, have to wait to see what comes out of the mitochondria and try to transform that. But when you bring them into the mitochondria, they’re better at competing with the pathways in there.”
The major news value is the findings could have many applications in metabolic engineering. There are many situations where it could be advantageous to confine all of the steps of a reaction in a small space, which may not only boost efficiency but also prevent harmful intermediates from drifting away and damaging the cell.
Yet we have to keep in mind that these heavier alcohols tend to be rather toxic in high concentrations. Making them inside the mitochondria, getting them past the cytoplasm and out of the cell is going to be quite a process on its own.
The MIT researchers are now trying to further boost isobutanol yields and reduce production of ethanol, which is still the major product of sugar breakdown in yeast.
Stephanopoulos takes up the explanation again saying, “Knocking out the ethanol pathway is an important step in making this yeast suitable for production of isobutanol. Then we need to introduce isobutanol synthesis, replacing one with the other, to maintain everything balanced within the cell.”
The third party commenter in the MIT press release is James Liao, a professor of chemical and biomolecular engineering at UCLA, who was not part of the research team, said, “So far, they have done a good job showing that this idea works. The next step is to see if this trend continues at a large scale and produces a high enough yield for commercial use.”
That’s the next mountain. Yeast today can make huge amounts of ethanol from sugar cane and corn. So far the outlook for tomorrow is the heavier and more desirable butanol and pentanol are looking better, but they’re not quite there yet.
The desirability of the heavy alcohols goes beyond the carbon atoms that would be recycled quickly through the carbon cycle. Pentanol carries 12, yes, twelve hydrogen atoms and butanol 9 hydrogen atoms. They are both great “hydrogen economy” formation, storage and fuel paths.
Comments
2 Comments so far


great work … but if they want to effect fossil fuel use then working on increasing the efficiency of the ICE would be a much better expenditure of brainpower …
please communicate