Feb
6
Lithium Battery Material Redesign For a Big Improvement
February 6, 2013 | Leave a Comment
SLAC National Accelerator Laboratory and Stanford University scientists set a new world record for energy storage, using a clever “egg yolk-shell” design to store five times more energy in the sulfur cathode of a rechargeable lithium-ion battery. The comparison is made directly to today’s commercial technology.
The performance is quite impressive beyond the capacity. The cathode also maintained a high level of performance after 1,000 charge/discharge cycles, paving the way for new generations of lighter, longer-lasting batteries for use in portable electronics and electric vehicles.
The team is led by Yi Cui, a Stanford associate professor of materials science and engineering and a member of the Stanford Institute for Materials and Energy Sciences, a SLAC/Stanford joint institute. The team published its results in Nature Communications.
Looking at the comparison shows today’s lithium-ion batteries typically retain about 80% of their initial capacity after 500 charge/discharge cycles. A doubling of the cycling performance will have a significant effect on the cost of batteries and the recycling expenses. The hassle of dealing with a declining battery would be twice as far out for consumers. It’s a major improvement.
Lithium-ion batteries release energy by moving lithium ions back and forth between two electrodes, the cathode and anode. Charging the battery forces the ions and electrons into the anode, creating an electrical potential that can power a wide range of devices. Discharging the battery – using it to do work – moves the ions and electrons to the cathode.
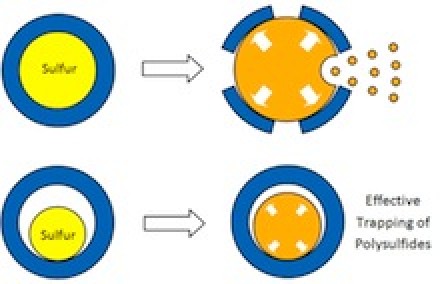
For some 20 years, researchers have known that sulfur could theoretically store more lithium ions, and thus much more energy, than today’s cathode materials. But two critical disadvantages prevented its commercial use: When lithium ions enter a sulfur cathode during discharging, they bond with sulfur atoms to create an intermediate compound that’s important for the cathode’s performance; but this compound kept dissolving, limiting the cathode’s energy-storage capacity. At the same time, the influx of ions caused the cathode to expand by about 80 percent. When scientists applied protective coatings to keep the intermediate compound from dissolving, the cathode would expand and crack the coating, rendering it useless.
That has held back the sulfur component progress. Sulfur is quite attractive as it isn’t a costly element, nor particularly dangerous.
Sulfur Egg Yolk Cathode Diagram. Image Credit: Zhi Wei She, Stanford University. Click image for more info.
Professor Cui’s innovation is a cathode made of nanoparticles, each a tiny sulfur nugget surrounded by a hard shell of porous titanium dioxide, like an egg yolk in an eggshell. Between the yolk and shell, where the egg white would be, is an empty space into which the sulfur can expand. During discharging, lithium ions pass through the shell and bind to the sulfur, which expands to fill the void but not so much as to break the shell. The shell, meanwhile, protects the sulfur-lithium intermediate compound from electrolyte solvent that would dissolve it.
Titanium dioxide is fairly common and low cost as well. It’s used in white paint, sunscreens and food coloring. The research is looking quite attractive now.
Each cathode particle is only 800 nanometers (billionths of a meter) in diameter, about one-hundredth the diameter of a human hair.
“It basically worked the first time we tried it,” Cui said. “The sulfur cathode stored up to five times more energy per sulfur weight than today’s commercial materials.
“After 1,000 charge/discharge cycles, our yolk-shell sulfur cathode had retained about 70 percent of its energy-storage capacity. This is the highest performing sulfur cathode in the world, as far as we know,” he said. “Even without optimizing the design, this cathode cycle life is already on par with commercial performance. This is a very important achievement for the future of rechargeable batteries.”
That’s quite an understatement; any alert battery manufacturer has to be intensely interested in this research.
This is only newest half of the story. For seven years, Cui’s group has demonstrated a succession of increasingly capable anodes that use silicon rather than carbon because it can store up to 10 times more charge per weight. Their most recent anode also has a yolk-shell design that retains its energy-storage capacity over 1,000 charge/discharge cycles.
There is much more to come – the lithium battery business could soon see a major breakthrough in applications. The question out there now is the processing, the costs, and if the sophisticated level of innovation that Cui is showing is applied there as well, we could see a new and larger plateau in the lithium battery market.


