Aug
23
Blow Up the Battery Materials Before You Build It
August 23, 2012 | 4 Comments
Take a paper like sheet of the world’s thinnest material, graphene, and blast it with a laser or a camera flash to blemish it with countless cracks, pores, and other imperfections and create an easy-to-make, quick-charging lithium-ion battery with high power density.
As a practical matter the graphene anode material can be charged or discharged 10 times faster than conventional graphite anodes used in today’s lithium-ion (Lith-ion) batteries.
Rechargeable Lith-ion batteries are the industry standard for cell phones, laptops and tablet computers, electric vehicles, and a range of other devices. Lith-ion batteries have a high energy density and can store large amounts of energy, but they suffer from a low power density meaning they are unable to quickly accept or discharge energy. The low power density is why it takes about an hour to charge your cell phone or laptop battery, and why electric automobile motors cannot rely on batteries alone and require a supercapacitor for high-power functions such as acceleration and braking.
Faster charge and discharge could reduce the need for the complex pairing of Lith-ion batteries and supercapacitors in electric vehicles, and lead to simpler, better-performing automotive engines based perhaps solely on high-energy, high-power Lith-ion batteries. This kind of battery improvement would also significantly shorten the time it takes to charge portable electronic devices and medical devices used by paramedics first responders and military personnel.
Koratkar and his team started investigating graphene to replace the graphite used today as the anode material, essentially a single layer of the graphite found commonly in our pencils or the charcoal we burn on our barbeques. This slow charging and discharging is because lithium ions could only physically enter or exit the battery’s graphite anode from the edges, and slowly work their way across the length of the individual layers of graphite.
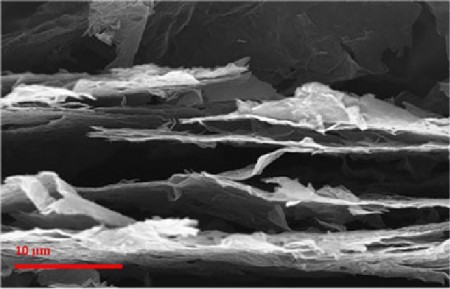
Graphene is an atom-thick sheet of carbon atoms arranged like a nanoscale chicken-wire fence. The team’s solution was to use a known technique to create a large sheet of graphene oxide paper. This paper is about the thickness of a piece of everyday printer paper, and can be made nearly any size or shape.
The research team then exposed some of the graphene oxide paper to a laser, and other samples of the paper were exposed to a simple flash from a digital camera. In both instances, the heat from the laser or photoflash literally caused mini-explosions throughout the paper, as the oxygen atoms in graphene oxide were violently expelled from the structure.
The aftermath of this oxygen exodus was sheets of graphene pockmarked with countless cracks, pores, voids, and other blemishes. The pressure created by the escaping oxygen also prompted the graphene paper to expand five-fold in thickness, creating large voids between the individual graphene sheets.
The researcher team quickly learned this blast damaged graphene paper performed remarkably well as an anode for a Lith-ion battery. Before treatment the lithium ions slowly traversed the full length of graphene sheets to charge or discharge. Now the ions used the cracks and pores as shortcuts to move quickly into or out of the graphene – greatly increasing the battery’s overall power density.
Koratkar’s team demonstrated how their experimental anode material could charge or discharge 10 times faster than conventional anodes in Lith-ion batteries without incurring a significant loss in its energy density. Despite the countless microscale pores, cracks, and voids that are ubiquitous throughout the structure, the graphene paper anode is remarkably robust, and continued to perform successfully even after more than 1,000 charge/discharge cycles. The high electrical conductivity of the graphene sheets also enabled efficient electron transport in the anode, which is another necessary property for high-power applications.
Koratkar and his team are confident their new battery, created by intentionally engineering defects in graphene, is a critical stepping-stone on the path to a fast charge – discharge battery. The process of making these new graphene paper anodes for Li-ion batteries can easily be scaled up to suit the needs of industry. The graphene paper can be made in essentially any size and shape, and the photo-thermal exposure by laser or camera flashes is an easy and inexpensive process to replicate.
The researchers have filed for patent protection for their discovery. The next step for this research project is to pair the graphene anode material with a high-power cathode material to construct a full battery.
The next step for this research project is to pair the graphene anode material with a high-power cathode material to construct a full battery.
Fast charging is going to really help the Lith-ion battery market and electrified product sales.
Comments
4 Comments so far


This is a great leap forward. Competitive process would build the nanostructure up one molecular layer at a time and that would be such an expensive process it would probably never be commercially feasible. But, just shredding the substrate on the back end is so much easier and cheaper – it might actually be commercially scaleable.
you’re in point of fact a excellent webmaster. The site loading pace is amazing. It seems that you’re doing any distinctive trick.
Moreover, The contents are masterpiece. you have performed a great task
in this subject!
Simply wanna comment on few general things, The website style and design is perfect, the content material is real superb : D.
I just want to mention I am just all new to blogs and seriously loved you’re blog. More than likely I’m planning to bookmark your website . You really come with perfect well written articles. Kudos for sharing your blog.