Apr
18
Super Enzyme Catalysts May Be Coming
April 18, 2012 | Leave a Comment
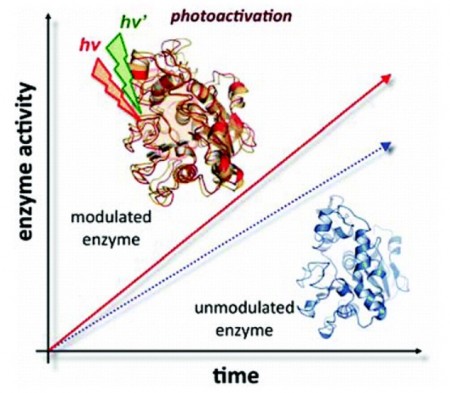
Pratul Agarwal leads a team at the Department of Energy’s Oak Ridge National Laboratory describing in a paper published in The Journal of Physical Chemistry Letters that light of specific wavelengths in an effect called photoactivation can be used to boost an enzyme’s function by as much as 8 to 52 fold – for effect, an 800 to 5200 percent improvement.
An average 30x increase in catalyst activity is no small matter. That potential could establish a new series of processes from biofuel to industrial chemicals to household items. Over a broad range of applications the impact would be very impressive.
The press release quotes Agarwal in describing how the idea of using light functions in nature.
“When light enters the eye and hits the pigment known as rhodopsin, it causes a complex chemical reaction to occur, including a conformational change,” Agarwal said. “This change is detected by the associated protein and through a rather involved chain of reactions is converted into an electrical signal for the brain.”
As everyone quickly realizes this happens very fast indeed.
Using that as a model, Agarwal’s team theorized that it should be possible to improve the catalytic efficiency of enzyme reactions by attaching chemical elements on the surface of an enzyme and manipulating them with the use of tuned light.
The team introduced a light-activated molecular switch across two regions of the enzyme Candida antarctica lipase B, or CALB – which breaks down fat molecules that was identified through modeling performed on DOE’s Jaguar supercomputer.
“Using this approach, our preliminary work with CALB suggested that such a technique of introducing a compound that undergoes a light-inducible conformational change onto the surface of the protein could be used to manipulate enzyme reaction,” Agarwal said.
While the researchers obtained final laboratory results at industry partner AthenaES, computational modeling allowed Agarwal to test thousands of combinations of enzyme sites, modification chemistry, different wavelengths of light, different temperatures and photo-activated switches. Simulations performed on Jaguar also allowed researchers to better understand how the enzyme’s internal motions control the catalytic activity.
The team’s work seems like a hyper speed evolutionary process.
“This modeling was very important as it helped us identify regions of the enzyme that were modified by interactions with chemicals,” said Agarwal, a member of ORNL’s Computer Science and Mathematics Division. “Ultimately, the modeling helped us understand how the mechanical energy from the surface can eventually be transferred to the active site where it is used to conduct the chemical reaction.”
Agarwal noted that enzymes are present in every organism and are widely used in industry as catalysts in the production of biofuels and countless other every day products. Researchers believe this finding could have immense potential for improving enzyme efficiency, especially as it relates to biofuels.
The team includes Christopher Schultz and Sheldon Broedel Jr. of AthenaES, Aristotle Kalivretenos of Aurora Analytics and Brahma Ghosh, an independent consultant.
A 30x average is going to be a major change. Even if a commercial version is only half as good, the change will be very significant. This stage isn’t prompting cost analysis, but the cost other than the enzymes themselves isn’t going to be a major issue unless moving the processed material along requires a rework – not a bad thing.
Still, the enzymes might turn out to be quite costly in the short term. But the opportunity is huge and cost cutting and more speed is sure to be on everyone agenda.
Congratulation to Agarwal and his team. A great insight, even greater results!