Aug
16
Fast Hydrogen Production Without Platinum
August 16, 2011 | 6 Comments
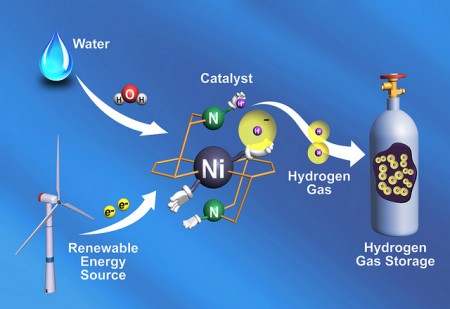
Researchers at the Pacific Northwest Lab (PNL) have used a common protein to guide the design of a material that can free hydrogen gas to store energy. The synthetic material works 10 times faster than the original source protein found in water-dwelling microbes.
A common microbe stores energy in the bonds of hydrogen gas with the help of a protein called a hydrogenase. Plants use photosynthesis to store the sun’s energy in chemical bonds, which other organisms use when they eat the plants as food. The PNL researchers wanted to pull out the active portion of the biological hydrogenase and redesign it into a catalyst with a stable chemical backbone.
The PNL researchers report in the August 12 issue of the journal Science, the synthesized catalyst clocks in at 100,000 molecules of hydrogen gas every second. That step is just one of a series of reactions to split water and make dihydrogen (H2) gas, but the researchers say the early result shows they can learn from nature how to control those reactions to make durable synthetic catalysts for energy storage.
Currently, the materials that spur reactions along called catalysts rely on expensive metals such as platinum. Coauthor Morris Bullock starts the explanation with, “This nickel-based catalyst is really very fast. It’s about a hundred times faster than the previous catalyst record holder. And from nature, we knew it could be done with abundant and inexpensive nickel or iron.”
In the study the researchers looked at only one small part of splitting water into hydrogen gas. Of the many steps, there’s one at the end when the catalyst has a hold on two hydrogen atoms that it has stolen from water and then snaps the two together making the H2 gas.
The catalyst does this by completely dismantling some hydrogen atoms from a source such as water and moving the pieces around. Due to the simplicity of hydrogen atoms, those pieces are positively charged protons and negatively charged electrons. The catalyst arranges those pieces into just the right position so they can be put together correctly. “Two protons plus two electrons equals one molecule of hydrogen gas,” says Bullock.
“We looked at the hydrogenase and asked what is the important part of this?” said Bullock. “The hydrogenase moves the protons around in what we call a proton relay. Where the protons go, the electrons will follow.”
Based on the hydrogenase’s proton relay, the experimental catalyst contained regions called “pendant amines” that dangled off the main structure and attracted protons. A pendant amine moves a proton into position on the edge of the catalyst, while a nickel atom in the middle of the catalyst offers a hydrogen atom with an extra electron (that’s a proton and two electrons for those keeping track).
The pendant amine’s proton is positive, while the nickel atom is holding on to a negatively charged hydrogen. Positioned close to each other, the opposites attract and the conglomerate solidifies into a molecule, forming the dihydrogen gas.
With that plan in mind, the team built potential catalysts and tested them. On their first try, they put a bunch of pendant amines around the nickel center, thinking more would be better. Testing their catalyst, they found it didn’t work very fast. An analysis of how the catalyst was moving protons and electrons around suggested too many pendant amines got in the way of the perfect reaction. An overabundance of protons made for a sticky catalyst, which pinched it and slowed down the hydrogen-gas-forming reaction.
Then the team trimmed a few pendant amines off their catalyst, leaving only enough to make the protons stand out, ready to accept a negatively charged hydrogen atom. The team found the newly trimmed catalyst performed much better than anticipated. At first they used conditions in which no water was present and the catalyst could create hydrogen gas at a rate of about 33,000 molecules per second. That’s much faster than their natural inspiration of hydrogenase, which clocks in at around 10,000 per second.
Most real-life applications will have water around, so the team added water to the reaction to see how it would perform. The catalyst ran three times as fast, creating more than 106,000 hydrogen molecules every second.
There remains an issue. The new catalyst isn’t very efficient. The catalyst runs on electricity – because it needs the electrons to pack into the chemical bonds – but it requires more electricity than practical, a characteristic called overpotential exists. While the overpotential is in place with a platinum catalyst, PNL is suggesting the new catalyst isn’t as efficient. How much more so isn’t made clear.
The feedstock isn’t straight water either. There’s a lot of amino acid (protein) involved. The hydrogen is coming out of the amino acid not the water – thus we’re wondering what the feedstock source of the hydrogen atoms might cost.
Yet, this is a major step. Platinum is expensive and prohibits developing water splitting for the hydrogen and is a barrier to economical fuel cells to recover the energy efficiently. Coming out with a partial step using low cost iron and nickel is very hopeful.
Bullock offers the team has some ideas on how to increase the efficiency. Also, future work will require assembling a catalyst that splits water in addition to making hydrogen gas. Even with a high electrical overpotential, the team sees the new catalyst with great prospects for future development.
When using water, even when a not so great electrical efficiency is the result, such screaming speeds will be very attractive. Especially if the capital cost is proportionally down to the price difference between platinum and nickel. A chemical store of energy using hydrogen, from cheap water splitting and cheap fuel cells would be new dynamic in personal to mid-size energy production
Comments
6 Comments so far


Nickel and Hydrogen seem to have a special relationship. Rossi and Blacklight both seem to be basing their work on this relationship as well.
Could these folks have stumbled onto Rossi’s secret catalyst for his e-cats?
It is interesting who is author of this article, even if the readers are not scientists, but rather interested in popular science they still deserve accurate and meaningful explanation. This raises another question whether the draft of the article was revised by staff of Pacific Northwest Lab (PNL), or Dr. Morris Bullock prior to putting it on web? The article is full of scientific “mambo-jumbo” and poetic expressions nothing to do with reality.
Platinum catalysts do not spur any similar to enzyme reaction, Pt. catalyst deposited on electrode oxidizes Hydrogen molecule H2 (containing two hydrogen atoms) into two Hydrogen ions commonly called Protons while this happens the electrode on which platinum catalysts is deposited losses two electrons stripped away.
Water is full of the hydrogen ions tightly bound in solvent in form of Hydronium ions and Hydroxide the number of Hydronium ions relative to Hydroxide determines pH of the water; less than 7 water is acidic, higher than 7 water is base solution.
There are many ways to produce hydrogen from water, simplest is to heat water above 2,000 C and it will disassociate to form Hydrogen gas and Oxygen gas, simple, but extremely inefficient. This what happened at Fukushima nuclear power station when water was heated in closed environment to very high temperature.
Another way to split (split here it is a correct term) is electrolysis of water two electrodes not necessarily fabricated from expensive platinum, water solution to which acid is added to improve ionic conductivity and ~240 kJ of electric power will do the splitting of 1 mole of water at normal conditions, i.e. normal pressure at sea level and normal summer temperature of 25C. The ~240 kJ will split 18 gram of water into ~16 and ~2 gram of Hydrogen gas. Not very efficient to drive hydrogen fueled cars, jets and cooking hotplates.
The ingenuity of the Pacific Northwest Lab (PNL) process borrowed Hydrogenase enzyme and embodied in the mimic of the enzyme is not based on water splitting since process results only in Hydrogen while Oxygen remains in water. The mimic of enzyme does not split water at all! As well as the catalyst does not …”holds on two hydrogen atoms that it has stolen from water and then snaps the two together making the H2 gas…” Nothing of that happens, I hope.
The Hydrogenase enzyme is capable of fetching protons from water and as its mimic it has Nickel-Iron center where fetched protons (hydrogen ions) are brought to the center, these two protons will remain protons for eternity unless the enzyme would receive two electrons. These two electrons (energy) must be supplied from outside. Either from windmill, or Sun radiation supplying stream of photons converted by a photosynthesis cell to electrons, or from thermal power station burning dirty coal converting heat to mechanical energy and to electrons.
To conclude water is not split borrowing from water hydrogen ions called protons makes water less acidic. Adding acid to water will add protons again keeping pH at desired level.
If however, somebody insists that hydrogen atoms are stolen from water by the novel Catalyst, mimic of the Hydrogenase enzyme the novel catalyst should be reported to federal police.
Why is hydrogen proving so illusive
We could use nuclear to dissociate H2 from O2 at high temperatures. Or even electricity for electrolysis. our problem is not a lack of H2 or conversion ability, it is the unwillingness of the immense, world girdling-(maybe ‘world strangling’ isd a better description), hydrocarbon mining industry. Coal and oil and gas are all “mining” the same “rock oil” or methane- “earth’s digestive gas”,(cows and people make it too), and coal which is like dried earth dung.
All these stuffs are incredibly dirty when burned.This is basic HS chemistry. We could have switched to clean and safe, non polluting nuclear but a few ignorant people could not tell the difference between a an A bomb and a Uranium steam generator and led,(watch “The Simpsons” cartoon as Homer routinely does the impossible by blowing up Springfield by pressing “the” red button on a control board at Burns’ plant, Always a button marked: “do not push”).
In fact, since the one or two accidental leaks and meltdowns-none ever melted the way to China), in America , nuclear power while being demonized has become as safe and sometimes safer than solar power.
With more new and re-built atomic plants, we would be able to use our hydrocarbons for plastics , chemicals and medicines. And where America leads, the rest of the world follows.
Unless someone invents high or room temperature superconductors, soon, or workable thermonuclear generation, or-the ultimate: a 40% or higher efficiency Photo Voltaic cell, nuclear power is the only way to save and regenerate an industrial economy without choking and cooking us all.
Yes, we could use nuclear to dissociate H2 & O2 at high temperatures. Or use electricity for electrolysis. our problem is not a lack of H2 or conversion ability, it is the unwillingness of the immense, world girdling-(‘world strangling’ may be a better description), hydrocarbon mining industry. Coal, oil and gas companies are all “mining” the same “rock oil”- methane- “earth’s digestive gas”,(cows and people make it too), and coal which is like dried earth dung.
All this goop and fizz are incredibly dirty when burned, this is basic HS chemistry. We could have switched to clean and safe, non polluting nuclear back in the 60’s, but a few ignorant people could not tell the difference between an A bomb and a Uranium powered steam generator, and led to cartoons(watch “The Simpsons” cartoon as Homer routinely does the impossible by blowing up Springfield by pressing “the” red button on a control board at Burns’ plant, Always a button marked: “do not push”), and to the idea that all nuclear plants can and might explode like A bombs.
In fact, since the one or two accidental leaks and meltdowns-none melted its’ way to China), in America and elsewhere, nuclear power, even when it was being demonized ,has become far cleaner and sometimes safer than solar power!
With more new and re-built atomic plants,(they do wear out-just like old battleships or oil tankers), we would be able to use our hydrocarbons for plastics , chemicals and medicines. And where America leads, the rest of the world follows.
Unless someone invents cheap high temp, or room temperature superconductors, or a “greater than 40%” or more, hi- efficiency Photo Voltaic cell, nuclear power and lots of it, is the only viable currently available way to save and regenerate an industrial economy ,quickly, without cooking us all. (I do wish Germany well but I doubt they’ll succeed and are already giving signs of imminent failure, as they abandon nuclear generation, out of absurd fear of tsunamis in the pacific ocean).
Burning Natural Gas rather than coal and oil is just substituting wine & beer for hard liquor and calling it a cure for alcoholism.