May
11
Solar Energy Directly to Hydrogen Production
May 11, 2011 | 2 Comments
Getting solar energy into a stored form is a major and elusive goal. One idea that has intrigued for decades is using solar energy to split water for the hydrogen product. Some wee progress has been made. May 10, 2011 saw a Swiss Ecole Polytechnique Fédérale de Lausanne (EPFL) team discovering that it is possible to protect hydrogen producing semiconductors with a uniform layer just a few nanometers thick.
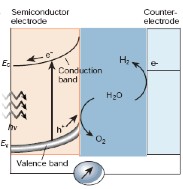
The current art has semiconductors to produce the energy as hydrogen, but the most efficient semiconductors are not the most stable. This knowledge explains why the EPfL work is significant. Photoelectrochemical cells (PEC) have been shown to directly split water into H2 and O2 (photoelectrolysis of water) thereby providing a basis for the renewable, clean production of hydrogen from sunlight. The team relies on a photoactive material the semiconductor, capable of harvesting and converting solar energy into stored chemical fuel, i.e. the hydrogen.
The process involves using a light-sensitive semi-conducting material such as cuprous oxide to provide the current needed to fuel the reaction. Although it is not expensive, the oxide is unstable if exposed to light in water.
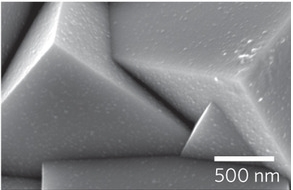
The research by PhD candidate Adriana Paracchino and Elijah Thimsen was published May 8, 2011 in the journal Nature Materials, and demonstrates that this problem can be overcome by covering the semiconductor with a thin film of atoms using the atomic layer deposition (ALD) technique.
With supervision by Professor Michael Grätzel in EPFL’s Laboratory of Photonics and Interfaces, the two young scientists achieved this remarkable feat by combining techniques used at industrial scale, and then applying them to the problem of producing hydrogen.
The team’s new process using the coated cuprous oxide can simply and effectively protect the semiconductor from contact with water, making it possible to use it as a photoelectrochemical solar cell. The advantages are numerous: cuprous oxide is abundantly available and inexpensive; the protective layer is completely impermeable, regardless of the roughness of the surface; and the process can easily be scaled up for industrial fabrication.
The team developed the technique by “growing” layers of zinc oxide and titanium oxide, one atom-thick layer at a time, on the cuprous oxide surface. By using the ALD technique, they were able to control the thickness of the protective layer down to the precision of a single atom over the entire surface. This level of precision guarantees the stability of the semiconductor while preserving all of its hydrogen-producing efficiency. The next step in the research will be to improve the electrical properties of the protective layer.
Using widely available materials and techniques that can be easily scaled up will help the “green” photoelectrochemical production of hydrogen meet the needs of industry.
What the press information leaves out is the key number – how efficient is it? While it might not be nice to blurt the question out, its key to industrial scale. A minimum around 10% of the visible spectrum would do to start.
The EPFL group grasps this with a website note that a 15% efficient solar cell feeding to a 70% efficient electrolyzer offers a total conversion efficiency of 10% obtainable under optimal illumination conditions. The U.S. Department of Energy has projected that an increase in efficiency of silicon solar cells to 20% would result in a hydrogen price of $8 per kilogram – something akin to gasoline fueled costs – back when gasoline was about $2.00 per gallon.
It’s a relief to see some progress in the field – a notoriously quiet field since the basic idea got some attention. The field still needs a very low cost fuel cell and a short to medium term storage solution or low cost transition to a light hydrocarbon or alcohol – but hydrogen as a fuel has always offered a very clean hope and attracts lots of attention. It’s just gotten another step closer and to the thoughtful mind an important one in a potentially very rich market.
Comments
2 Comments so far


We are intrusted in this Technology.Please let us know following points:
1.Is this commercialy available.If yes, pl.provide the details.
2.What would be the cost.
What’s up every one, here every one is sharing these familiarity, so it’s fastidious to read this website, and I used to visit this web
site daily.