Mar
22
A New Battery Structure Revolution for Better Performance
March 22, 2011 | 3 Comments
Professor Paul Braun has been leading a research group at the University of Illinois at Urbana-Champaign reporting a significant breakthrough in the development of battery technology. The research group has found a way to enable fast charging and discharging of electrical power while retaining high-energy storage capacity.
Here’s the problem, rapidly charging and discharging rates are an important feature of electrical energy storage devices, but doing it quickly causes dramatic reductions over cycles in the energy that can be stored or delivered by most any commercial rechargeable battery. Ultra or supercapacitors charge very quickly with almost endless cycles but are restricted to much lower amounts of stored energy per mass (energy density) than batteries.
That put the Illinois team on the hunt for a storage technology that combines the charge rate performance of supercapacitors with the energy density of batteries to significantly advance portable and distributed power technology.
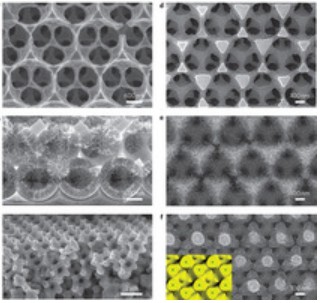
The research result is very fast battery charge and discharge rates with minimal capacity loss by using cathodes made from a self-assembled three-dimensional bicontinuous nanoarchitecture consisting of an electrolytically active material sandwiched between rapid ion and electron transport pathways.
Now for some explaining. The team designed a self-assembling three-dimensional nanostructure for battery cathodes in which tiny spheres are packed onto a surface and then form automatically into a lattice like structure. Once they are in place, metal is packed around the spheres, which are then melted, leaving a metal sponge-like 3-D scaffolding. It sounds a lot like nano-investment casting.
The new spaces within the metal scaffolding are enlarged using an “eleoctropolishing” technique and then filled with a thin-film active material.
One very interesting point, the structure has been used successfully in both Nickel Metal Hydride and Lithium-ion batteries and the Illinois group says that it can be used with any battery material applied to the metal frame.
Professor Braun says, “This system that we have gives you capacitor-like power with battery-like energy. Most capacitors store very little energy. They can release it very fast, but they can’t hold much. Most batteries store a reasonably large amount of energy, but they can’t provide or receive energy rapidly. This does both.”
Here’s the brave new world quote, “If you had the ability to charge rapidly, instead of taking hours to charge the vehicle you could potentially have vehicles that would charge in similar times as needed to refuel a car with gasoline,” said Braun. “If you had five-minute charge capability, you would think of this the same way you do an internal combustion engine. You would just pull up to a charging station and fill up.”
Braun’s groups thin film wrapped into three-dimensional structure, achieves both high active volume (high capacity) and large current. They have demonstrated battery electrodes that can charge or discharge in only a few seconds, making the new design 10 to 100 times faster than equivalent bulk electrodes, yet the design can perform normally in existing devices.
The best news is all of the processes the group used are also used at large scales in industry so the technique could be scaled up for manufacturing.
The key to the group’s novel 3-D structure is the sphere’s self-assembly. Trying to create such a uniform lattice by other means is time-consuming and impractical, but the inexpensive spheres settle into place automatically. Now, that is just marvelous serendipity. Lets hope the sphere’s self-placement will duplicate at scale.
It would be remiss not to double up on one point – as Braun put it, “We like that it’s very universal, so if someone comes up with a better battery chemistry, this concept applies. This is not linked to one very specific kind of battery, but rather it’s a new paradigm in thinking about a battery in three dimensions for enhancing properties.”
The question not directly answered in the university press release or the study, is the effect on total number of charge discharge cycles or cycle life. One would assume that the cycle count would go up, but without testing, and perhaps with the rush to publish the count is underway, this has to be answered.
It seems today that there can be an answer to the dilemma of electron storage. With an ‘all quiet’ on the Eestor front, this news from Illinois is very welcome indeed.
Say a battery electric vehicle needs 4 hours or 240 minutes for a full charge. Cutting that by a factor of ten, the low range predicted above, a recharge would only take 24 minutes. At the high range the wait would be only 2.4 minutes. At 2.4 minutes, even at a one hundred mile driving range, a 2.4 minute wait would change the perspective making EV adoption much more interesting.
This looks really good. Here’s the published study link at Nature Nanotechnology.
Comments
3 Comments so far



Using Carbon foam allowed Firefly Energy to achieve similar ends. In the Firefly battery they also demonstrated an increased number of charge/discharge cycles with lead acid chemistry.
[…] groep onderzoekers heeft een manier bedacht waarmee batterijen aanzienlijk sneller zijn op te laden. De opslagcapaciteit van de batterijen gaat […]
[…] groep onderzoekers heeft een manier bedacht waarmee batterijen aanzienlijk sneller zijn op te laden. De opslagcapaciteit van de batterijen gaat […]