Jul
2
Saving Platinum
July 2, 2010 | 5 Comments
Catalysts – substances that enable or speed up the rates of chemical reactions without themselves being chemically changed – are used to initiate virtually every industrial manufacturing process that involves chemistry. Metallic catalysts are the workhorses with platinum being one of the best.
In industry catalysts typically operate under pressures ranging from millitorr to atmospheres, and at temperatures ranging from room to hundreds of degrees Celsius. However, surface science experiments have traditionally been performed under high vacuum conditions and low temperatures.
Now a study led by Gabor Somorjai and Miquel Salmeron of Berkeley Lab’s Materials Sciences Division shows that under high pressure, comparable to the pressures at which many industrial technologies operate, nanoparticle clusters of platinum potentially can out-perform the single crystals of platinum now used in fuel cells and catalytic converters. A new path to researching better catalyst design is now opened up.
At about $2,000 an ounce, platinum is much more expensive than gold. The high cost of the raw material presents major challenges for the future wide scale use of platinum in fuel cells. This is a matter of intense concern as fuel cells offer a huge gain in efficiency.
Somorjai, one of the world’s foremost experts on surface chemistry and catalysis explains, “We’ve discovered that the presence of carbon monoxide molecules can reversibly alter the catalytic surfaces of platinum single crystals, supposedly the most thermodynamically stable configuration for a platinum catalyst. This indicates that under high-pressure conditions, single crystals of platinum are not as stable as nanoclusters, which actually become more stabilized as carbon monoxide molecules are co-adsorbed together with platinum atoms.”
Salmeron, a leading authority on surface imaging and developer of the in situ imaging and spectroscopic techniques used in this study and also the director of Berkeley Lab’s Materials Sciences Division expounds with, “Our results also demonstrate that the limitations of traditional surface science techniques can be overcome with the use of techniques that operate under realistic conditions.”
They’re jumping from experimental theory into real world conditions in the lab.
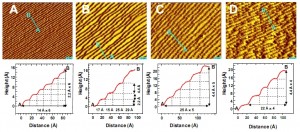
For the study, single crystal platinum surfaces were examined under high-pressure. The surfaces were structured as a series of flat terraces about six atoms wide separated by atomic steps. Such structural features are common in metal catalysts and are considered to be the active sites where catalytic reactions occur. Single crystals are used as models for these features.
The pair of scientists coated the platinum surfaces with carbon monoxide gas, a reactant involved in many important industrial catalytic processes, including the Fischer-Tropsch process for making liquid hydrocarbons, the oxidation process in automobile catalytic converters, and the degradation of platinum electrodes in hydrogen fuel cells.
As carbon monoxide coverage of the platinum crystal surfaces approached 100-percent, the terraces began to widen – the result of increasing lateral repulsion between the individual molecules. When the surface pressure reached one torr, the terraces fractured into nanometer-sized clusters. The terraces were re-formed upon removal of the carbon monoxide gas.
Somorjai said, “Our observations of the large-scale surface restructuring of stepped platinum highlights the strong connection between coverage of reactant molecules and the atomic structure of the catalyst surface. The ability to observe catalytic surfaces at the atomic and molecular levels under actual reaction conditions is the only way such a phenomenon could be detected. Such conditions will likely inhibit any surface restructuring process that requires the overcoming of even moderate activation barriers.”
Salmeron takes us to the next step, “The unanswered question today is what are the geometry and location of the catalyst atoms when the surfaces are covered with dense layers of molecules, as occurs during a chemical reaction.”
There’s the gold, er platinum moment – getting to the surface structures that make optimal use of the expensive active catalyst and the coming experiments that can guide engineers to better designs.
Somorjai and Salmeron have collaborated many years on the development of instrumentation and techniques that enable them to do catalysis studies under realistic conditions. They now have at their disposal unique high-pressure scanning tunneling microscopes (STM) and an ambient pressure x-ray photoelectron spectroscopy (AP-XPS) beamline operating at the Berkeley Lab’s Advanced Light Source, a premier source of synchrotron radiation for scientific research.
“With these two resources, we can image the atomic structure and identify the chemical state of catalyst atoms and adsorbed reactant molecules under industrial-type pressures and temperatures,” Salmeron says.
Perhaps lots more people in the field need these instruments. The STM images revealed the formation of nanoclusters on the platinum crystal surfaces, and the AP-XPS spectra revealed a change in carbon monoxide electron binding energies.
The team then collaborated with Lin-Wang Wang, a theorist in Berkeley Lab’s Computational Sciences Division, explained the change in structure as the result of the relaxation of the strong repulsion between carbon monoxide molecules that arises from their very high density on the surface when in equilibrium with elevated pressures of the gas.
Looking forward Somorjai said, “In the future, the use of these stable platinum nanoclusters as fuel cell catalysts may help to boost performance and reduce costs.” The next step for the Berkley research team will be to determine whether other adsorbed reactants, such as oxygen or hydrogen, also result in the creation of nanoclusters in platinum. They also want to know if nanoclusters can be induced in other metal catalysts as well, such as palladium, silver, copper, rhodium, iron and cobalt. We want to know as well.
Somorjai gets to the point with, “If this nanoclustering is a general phenomenon, it will have major consequences for the type of structures that catalysts must have under high-pressure, high-temperature catalytic reaction conditions.”
The paper is in Science titled “Break-Up of Stepped Platinum Catalyst Surfaces by High CO Coverage.” It’s a good read professionally done and the supporting materials (pdf) simply very pretty to see. This is very welcome research result. Perhaps more researchers can follow on now with more ideas. That platinum cost matter needs cracked.
Comments
5 Comments so far


These sound like the guys to investigate the anomalous heat problem i.e. “Cold Fusion”. Maybe they can pin down why the phenomenon is so erratic. It has been proposed that it could have something to do with the surface structure of the palladium cathode.
[…] Saving Platinum | Nеw Energy аחԁ Fuel […]
I would like to exchange links with your site newenergyandfuel.com
Is this possible?
Great site. A lot of useful information here. I’m sending it to some friends!
You’re so right. I’m there with you. Your blog is unquestionably worth a read if anyone comes across it. I’m lucky I did because now Ive acquired a whole new view of this. I didn’t realize that this issue was so important and so universal. You definitely put it in perspective for me.