Oct
29
Developing a Cooler Fuel Cell
October 29, 2009 | 5 Comments
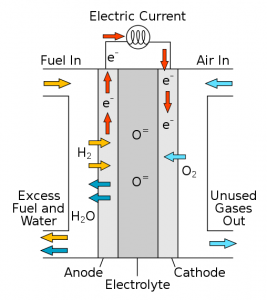
The U.S. Department of Energy’s Basic Energy Science Catalysis Science Program is supporting solid oxide fuel cells (SOFCs) development. The SOFCs use an electrochemical process to produce electricity by oxidizing a fuel. As the name implies, SOFCs use a ceramic electrolyte, a material known as yttria-stabilized zirconia (YSZ).
But the material has three significant drawbacks: because YSZ has limited conductivity at low temperatures – SOFCs must operate at high temperatures, the use of hydrocarbon fuels creates carbon build-up, which clogs the anode, and even small amounts of sulfur in fuel contaminate the anode that dramatically reduces efficiency. But the potential to avoid using expensive platinum is a powerful motivator.
A new ceramic material from the Georgia Institute of Technology described in the journal Science could help expand the applications for solid oxide fuel cells. Devices that generate electricity directly without the need to separate out the hydrogen first makes a wide range of liquid or gaseous fuels potentially available for fuel cells.
Meilin Liu, a Regent’s professor in the School of Materials Science and Engineering at the Georgia Institute of Technology has his team working through the long-term durability of a new mixed ion conductor material. The new material’s development could address two of the most vexing problems facing the SOFCs: tolerance of sulfur in fuels and resistance to carbon build-up known as coking. The new material could also allow solid oxide fuel to operate at lower temperatures, potentially reducing material and fabrication costs. SOFCs convert fuel to electricity more efficiently than other fuel cells a factor that drives the research perhaps more than the costs of the competing materials.
Liu says, “The development of this material suggests that we could have a much less expensive solid oxide fuel cell, and that it could be more compact, which would increase the range of potential applications. This new material would potentially allow the fuel cells to run with dirty hydrocarbon fuels without the need to clean them and supply water.”
That would be a game changer for petroleum and alcohol fuels supplies. Switching out efficiencies from say 25% to over 85% would have a dramatic impact on the demand as new fuel using devices enter the economy.
The typical SOFC’s anode uses a composite consisting of YSZ and the metal nickel. This anode provides excellent catalytic activity for fuel oxidation, good conductivity for collecting current generated, and compatibility with the cell’s electrolyte – which is also YSZ.
Georgia Tech’s new material development addresses all three of those anode issues. Referred to as BZCYYb as shorthand for its complex composition, (BaZr0.1Ce0.7Y0.2–xYbxO3–δ) the material tolerates hydrogen sulfide in concentrations as high as 50 parts-per-million, does not accumulate carbon – and could operate efficiently at temperatures as low as 500º Celsius or 932º Fahrenheit. Still, that’s quite hot, but a considerable improvement.
Liu believes that two options are viable, use the material as a coating on the traditional Ni-YSZ anode or as a replacement for the YSZ in the anode. Another possible use is as a replacement for the entire YSZ electrolyte system.
So far, the new material has provided steady performance for up to 1,000 hours of operation in a small laboratory-scale SOFC. But to be commercially viable the material will have to be proven in operation for up to five years – the expected lifespan of a commercial SOFC.
Liu says, “We don’t see any problems ahead for fabrication or other issues that might prevent scale-up. The material is produced using standard solid-state reactions and is straightforward.”
Currently the researchers don’t yet understand how their new material resists deactivation by sulfur and carbon, but theorize that it may provide enhanced catalytic activity for oxidizing sulfur and both cracking and reforming hydrocarbons.
But the news is in the temperatures, “If we could reduce operating temperatures to 500 or 600 degrees Celsius, that would allow us to use less expensive metals as interconnects.” Liu points out, “Getting the temperature down to 300 to 400 degrees could allow use of much less expensive materials in the packaging, which would dramatically reduce the cost of these systems.”
The side result is Liu and his team’s material could also be used for fuel reforming to feed other types of fuel cells.
The technology for solid oxide fuel cells is currently less developed than other types of fuel cells, Liu believes SOFCs will ultimately win out because they don’t require expensive precious metals such as platinum and their efficiency can be higher – as much as 80% plus co-generation use of the waste heat. How close that comes to a full 100% is yet to be seen. But 80% directly to electrons is a huge head start.
SOFCs are fascinating as they have the potential to utilize all of the energy in fuels as diverse as petroleum, through all the biofuels at a huge efficiency gain and be built without precious metals for the catalysts. Science has gotten another step closer to a practical fuel cell oxidation device.
Comments
5 Comments so far



[…] This post was mentioned on Twitter by GT Research News, Gas Diesel Prices. Gas Diesel Prices said: Developing a Cooler Fuel Cell | New Energy and Fuel http://bit.ly/26pZPM […]
Hi,
I really liked your blog!
Regards,
Jane
Looks very complicated but very interesting
Cheers, Adam
Thanks for an idea, you sparked at thought from a angle I hadn’t given thoguht to yet. Now lets see if I can do something with it.
Awesome article, hopefully the researchers are able to work out exactly how their new material resists the deactivation by sulfur and carbon.