Oct
28
Fuel Cell Progress – A Better Way to Burn
October 28, 2009 | 2 Comments
Oxidizing fuels is problematic because combustion is so inefficient at producing usable energy. For example, when gasoline is used to power a vehicle, at least 80 percent of the energy produced is wasted as heat. That’s reversed with vehicles that run on electricity. Better than 80 percent of the energy supplied to the vehicle is converted into motion, with only 20 percent lost as heat. Many other combustion devices as well could be replaced with electricity or with electricity produced by fuel cells.
Fuel cells are devices that produce electricity from simple fuels from hydrogen up to the simple hydrocarbons and alcohols, without burning them, so fuel cells are a very desirable and promising technology. Using light gases and liquid fuels could power everything from cars and homes on to small portable devices such as cellphones and laptops. But fuel cells come at a very high cost due to the materials and construction costs, thus researchers have been trying to find ways to make the devices less expensive.
One group, an MIT team led by Associate Professor of Mechanical Engineering and Materials Science and Engineering Yang Shao-Horn with researchers at the Japan Institute of Science and Technology, and the Brookhaven National Laboratory has found a form of fuel cell electrode that promises to dramatically increase the efficiency of the electrodes in the type of fuel cell that uses methanol instead of hydrogen as its fuel and is considered a top candidate as a replacement for batteries in portable electronic devices. The MIT electrodes are made of platinum, increasing their efficiency means that much less of the expensive metal is needed to produce a given amount of power. Moreover, methanol is something that could be produced in huge quantities.
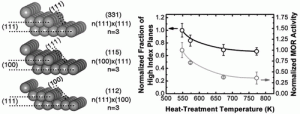
The MIT team found a key to the boost in efficiency is to change the surface texture of the platinum material. Instead of leaving it smooth, the researchers gave it tiny stairstep shapes. That adaptation approximately doubled the electrode’s ability to catalyze oxidation of the fuel and thus produce electric current. The team believes that further development of the surface structures could end up producing far greater increases, yielding more electric current for a given amount of platinum. That is critical, as the platinum raw material is wildly expensive.
Shao-Horn says, “One of our research focuses is to develop active and stable catalysts,” and this new work is a significant step toward “figuring out how the surface atomic structure can enhance the activity of the catalyst” in direct methanol fuel cells.
For the experiments, the team used platinum nanoparticles deposited on the surface of multi-wall carbon nanotubes. Chemical engineering graduate student Seung Woo Lee says that many people have been experimenting with the use of platinum nanoparticles for fuel cells, but the results of the particle size effect on the activity so far have been contradictory and controversial. “Some people see the activity increase, some people see a decrease” in activity as the particle size decreases. “There has been a controversy about how size affects activity.”
The new experiments show that the key factor is not the size of the particles, but the details of their surface structure. Mechanical engineering postdoctoral researcher Shuo Chen says, “We show the details of surface steps presented on nanoparticles and relate the amount of surface steps to the activity,” By producing a surface with multiple steps on it, the team doubled the activity of the electrode.
The team members are now working on creating surfaces with even more steps to try to increase the activity further. Theoretically, they’re saying, it should be possible to enhance the activity by orders of magnitude. That’s a very bold suggestion, an order of magnitude improvement would be stunning, going up from there would be at the edge of astonishing.
Shao-Horn suggests that the key factor is the addition of the edges of the steps, which seem to provide a site where it’s easier for atoms to form new bonds. The construction of the steps creates more of those active sites. Additionally and quite importantly, the team has shown that the step structures are stable enough to be maintained over hundreds of cycles. Stability over a long term, over tens of thousands of cycles is going to be key for developing practical and cost effective direct methanol fuel cells.
The research team also hopes to understand whether the steps enhance the oxygen reduction part of the process that takes place in a fuel cell. So far study has looked at the enhancement of oxidation. The question, “Does the addition of steps to the surface also enhance the oxygen reduction” Shao-Horn says, “We need to find why it does, or why it doesn’t.” The team expects to have answers to that oxygen reduction matter in the next few months.
The results are reported Oct. 13 in the Journal of the American Chemical Society showing a linear relationship between the intrinsic activity and the amounts of surface steps. Increasing surface steps on Pt nanoparticles of ~2 nm led to enhanced intrinsic activity up to 200% (current normalized to Pt surface area) for electro-oxidation of methanol.
Fuel to combustion yielding heat and pressure thus converted to mechanical energy is handy, well understood and in wide use, but such an inefficient set of steps leaves a huge amount of energy unused. Oxidation through a fuel cell jumps over several steps that need eliminated for efficiency. The MIT, the Japan Institute of Science and Technology, and the Brookhaven National Laboratory group is showing that there are other paths with great potential. Perhaps the structure of the catalyzing platinum will work on the other catalysts in research.
After months of quiet on the fuel cell front – this is great news.
Comments
2 Comments so far


Естественно как ни крути вы все правильно написали в своей записи Виагра помогает
Виагра мечтай
может даже больше чего ожидали.
Useful blog website, keep me personally through searching it, I am seriously interested to find out another recommendation of it.