Aug
6
A Battery So Good and Its Real
August 6, 2009 | 15 Comments
Japan’s National Institute of Advanced Industrial Science and Technology (AIST) researchers are developing a lithium-air cell with a new structure (a set of three different electrolytes) to avoid the degradation and performance problems of conventional lithium-air cells. But the big news is in the discharge capacity.
The newly developed lithium-air cell has shown a continuous cathode discharge capacity of 50,000 mAh g-1 per unit mass of the carbon, catalyst and binder. Compare that to conventional Li-ion batteries that offer 120-150 mAh g-1 active material + conduction assisting carbon + binder, and conventional lithium-air cells that offer 700-3,000 mAh g-1. This is a massive improvement in total power.
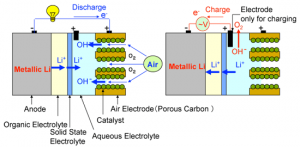
Lithium-air cells, dispense with the intercalation cathode of lithium-ion batteries and use a catalytic air cathode in combination with an electrolyte and a lithium anode instead. Lithium-air cells are attractive because of their theoretically very high energy capacity, but one of the serious problems with lithium-air cells reported to date is that a solid reaction product (Li2O or Li2O2), which is not soluble in organic electrolyte, clogs the air electrode (cathode) in the discharge process. If the air electrode is fully clogged, O2 from atmosphere cannot be reduced any more.
The AIST researchers use an organic electrolyte on the anode (metallic lithium) side and an aqueous electrolyte on the cathode (air) side. The two electrolytes are separated by a solid state electrolyte lithium super-ion conductor glass film, LISICON so that the two electrolytic solutions do not intermix. Only lithium ions pass through the solid electrolyte, and the battery reactions proceed smoothly.
AIST confirmed that the discharge reaction product is not a solid substance like lithium oxide (Li2O) seen in conventional batteries, but lithium hydroxide (LiOH). LiOH dissolves in the aqueous electrolyte so clogging of the pores does not occur at the carbon cathode. Water and nitrogen do not pass through the solid electrolyte at the partition wall, there are no unwanted reactions with the metallic lithium anode. During charging, corrosion and degradation of the air electrode is prevented by using another cathode electrode exclusively for charging. Very clever, dividing the cells into sections where the dividers only allow the communication for optimum performance.
Metallic lithium is used as the anode, and an organic electrolyte containing lithium salt is used on the anode side. A lithium-ion solid electrolyte is placed in between the two electrolytic solutions as a partition wall to separate the cathode and anode sides. An alkaline water-soluble gel is used as the aqueous electrolyte for the cathode side and the cathode consists of porous carbon and an inexpensive oxide catalyst. This information does lead one to wonder about the operating temperatures.
The discharging reactions proceed as follows:
1. Reaction at the anode: Li→ Li+ + e-
Lithium ions dissolve into the organic electrolyte as lithium ions (Li+) and the electrons are fed into the conductor wire. The dissolved lithium ions (Li+) pass through the solid electrolyte into the aqueous electrolyte on the cathode side.
2. Reaction at the cathode: O2 + 2H2O + 4e- → 4OH-
Electrons are fed from the conductor wire, and oxygen from the air and the reduction reacts on the surface of catalyst in the porous carbon to produce hydroxyl ions (OH-). They meet with lithium ions (Li+) in the aqueous electrolyte and produce water-soluble lithium hydroxide (LiOH).
The charging reactions proceed as follows:
1. Reaction at the anode: Li+ + e- → Li
Electrons are fed from the conductor wire, and lithium ions (Li+) in the aqueous electrolyte of the cathode side pass through the solid electrolyte and reach the surface of the anode where metallic lithium precipitates.
2. Reaction at the cathode: 4OH- → O2 + 2H2O + 4e-
Oxygen gas is generated. Generated electrons are fed to the conductor wire.
The new lithium-air batteries allow for continuous operation if the aqueous electrolyte on the cathode side is exchanged and metallic lithium is resupplied to the anode, say by means of exchangeable cassettes. The researchers say that this concept could then be taken as a “lithium fuel cell.” By retrieving LiOH from the aqueous electrolyte in the air electrode, metallic lithium can be recovered easily and reused as the fuel.
The opportunity for automotive use is huge; the researchers suggest that the technology holds great potential. For example at a filling station, the driver of a vehicle so equipped could exchange the aqueous electrolyte for the air electrode and refill the metallic lithium for the anode in the form of cassettes, and then continue driving without waiting for batteries to be recharged. That could be very quick indeed.
AIST says that the new lithium-air battery needs further technical improvement toward practical use.
Generally, there are two directions in this new lithium-air battery research, one is for a rechargeable lithium air battery and the other is for a lithium fuel cell. Understanding the difference might not seem important, but a battery would only need to be plugged in to a charger whereas a fuel cell would only need a change out of the modules or cassettes. Time will tell which would be most customer friendly and how they might be priced.
The discharge rate seems nearly feasible for automotive use. There would need to be some fast discharge capability for acceleration suggesting a capacitor. But .1A/g is not so low as to be impractical. What is important is the air cell design is at least three times high than the very best LithIon batteries of today.
The lifespan or the total cycles is also of interest, but not addressed in the report. Even so, the module or cassette exchange might well solve that matter a well with a pattern of a charges followed by and exchange of modules. Its easy to see this might work in a big way. Moreover the supply of lithium would eb greatly extended and subject to endless recycling.
This is worthwhile news. There is some speculation that its on Toyota’s mind as well, when representative were talking some months ago when they mentioned that current lithium batteries would be replaced by greatly superior technology units within a few years.
EEStor it seems won’t be alone in the high capacity electron storage market.
Comments
15 Comments so far


Having to dump electrolyte and load metallic lithium will kill this technology. It’s too much like having to ‘buy gas’ for your electric car. Consumers will never go there.
a solution any solution better than no solution, brillient. but there is competion, which adds diversity and can only mean good.diverse out product cars phones etc. storage, all systems go.
This may be a better fit for long-haul trucks than cars. Truck stops would re-fill/exchange canisters. The trucks would carry a large battery to travel long distances, but not require as high discharge/weight as cars.
How much cheaper is electricity than desiel fuel? The environmental improvement would be enormus.
I don’t really understand this article, or the original.
I am not technically trained however, so perhaps someone would explain further.
‘a continuous cathode discharge capacity of 50,000 mAh g-1 per unit mass of the carbon’
Presumably this refers to power output, as opposed to energy density. Do we know anything about the latter? Electric cars already seem to be able to accelerate acceptably.
My confusion gets worse when it is said:
‘The discharge rate seems nearly feasible for automotive use. There would need to be some fast discharge capability for acceleration suggesting a capacitor. But .1A/g is not so low as to be impractical. What is important is the air cell design is at least three times high than the very best LithIon batteries of today.’
??:Lithium Ion batteries are already in use in cars, and accelerate fine.
The main problem is energy density, not power density, and from what I can see this makes the battery enormously more power dense without helping with energy density.
Great site, BTW – I have put it into my favourites.
Perhaps you would list some contact details so we can alert you to new news stories, other sites you might like to be listed by and list in your blogroll and so on
Hi David,
mAh per gram is milli amps coming in an hour from unit of mass. Simply total up the grams you’d need to get your maximum demand. So David’s assumption about “power output” is correct, in a general way. As this is early research results, general is what we’re getting.
Density as a loose description gives a sense of the energy in a mass without the release rate as part of the equation. Density is a bit like the amount of electrons in an amount of something. A lot is known about density, it’s the density availability over time and the impact that release rates have on the density that drive researchers a little crazy.
I’m fully to blame for the David’s confusion. The .1A/gram is a fuel cell number, not the battery as best I can tell from the original article and failed to re note that fact in the paragraph.
Battery performance metrics can get very confusing. Total energy per mass isn’t really a “performance” metric as it doesn’t express how fast electrons come out or the impact discharges have on the available total. Using common metrics isn’t going to help much either, the “Cold Cranking Amps” we use when choosing a lead acid starting battery isn’t going to do a EV storage buyer much good. Those battery designs aren’t meant to deliver the electrons over time at a variable rates.
The researchers in this piece have a success in delivery improvement from amazing density. Its a big one. But the development has a way to go. The direction development goes likely comes from the best uses that experiments reveal.
So . . . maybe a rechargable EV battery is coming from this breakthrough, maybe it will be a fuel cell design. But a certainty is other researchers are likely going to segment the ‘battery or fuel cell’ as results here suggest. Which means that both directions are going to get attention – and that the effort in Japan is worldwide news worthy.
Battery chemistry can be a bewildering field for making sense of the metrics. And the customary metrics usually leave those not in the business puzzled. Charts using temperature, charge/discharge rates, impacts on total power availability can be found, but that would be for readily available units for sale. Lithium air simply isn’t there yet.
But by mainstream measures of the field, these are spectacular laboratory results.
BW
I recently came across your blog and have been reading along. I thought I would leave my first comment. I don’t know what to say except that I have enjoyed reading. Nice blog. I will keep visiting this blog very often.
Susan
I’ve recently started a blog, the information you provide on this site has helped me tremendously. Thank you for all of your time & work.
This post makes a lot of sense !
I was just having a conversation over this I am glad I came across this it cleared some of the questions I had.
I’ve been checking your blog for a while now, seems like everyday I learn something new 🙂 Thanks
Hello, this is my first time i visit here. I found so many interesting in your blog especially on how to determine the topic. keep up the good work.
Awesome post. I so good to see someone taking the time to share this information
Nice post! You truly have a wonderful way of writing which I find captivating! I will definitely be bookmarking you and returning to your blog. In fact, your post reminded me about a strange thing that happened to me the other day. I’ll tell you about that later…
Thanks for posting. Good to see that not everyone is using RSS feeds to build their blogs 😉
Very interesting topic , thanks for putting up.