Oct
27
Fuel From Thin Air
October 27, 2008 | 10 Comments
Headlines like that really set off skeptical responses. But nature is doing it and has been at it for at least hundreds of millions of years. Cleaving off the O2 from water made animal life possible. It exists so it’s only a matter of time until science comes up with ways to do it too.
There are three major efforts underway. Scandia National Lab’s Fuels and Energy Transitions Department has a project that works albeit likely with a high-energy input cost. Newcastle University might be a little closer with a catalyst approach that yields carbon products called cyclic carbonates, a precursor to many liquid carbon compounds that could be fuels or made into common fuels. The catalyst is used in a chemical reaction so the energy input is greatly reduced. It’s the energy inputs that are the challenge; the CO2 supply near power plants, cement producers and other emitters is abundant. There are several efforts to “cleanse” CO2 from the air, which may also yield feed stock sources.
The likely top of the list of candidates to get airborne or effluent CO2 reformed to fuels is Carbon Sciences. “We are very excited about our novel process to transform CO2 into fuel,” says company CEO Derek McLeish. “Based on our research to date, we believe that we will be able to demonstrate our technology within the next several months with a prototype that can convert a stream of CO2 into an immediately flammable liquid fuel.”
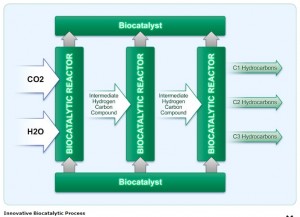
The technology uses CO2 to create methane, ethane, and propane, the three simplest hydrocarbons that can be used to make high-grade gasoline and other fuels. The key to the process is “biocatalysis,” a process where natural catalysts are used to perform chemical reactions. Biocatalysis is said to be a more energy efficient and cost-effective way to break down CO2, making the possibility of a large-scale ramp up economically feasible.
Dr. Naveed Aslam, the company’s chief technology officer and inventor of the process explains the approach uses a low energy biocatalytic hydrolysis process that splits water molecules into hydrogen atoms and hydroxide ions. The hydrogen is used to create hydrocarbons, while the free electrons in the hydroxide are used for fueling the biocatalytic process. Dr. Aslam says. The process “is based on natural organic chemistry processes that occur in all living organisms where carbon atoms, extracted from CO2, and hydrogen atoms extracted from H2O, are combined to create hydrocarbon molecules using biocatalysts and small amounts of energy.”
The key is the “using small amounts of energy.”
The Carbon Science’s webpage on the technology states, “By innovating at the intersection of chemical engineering and bio-engineering, we have discovered a low energy and highly scalable process to transform large quantities of CO2 into gaseous and liquid fuels using organic biocatalysts. The key to our CO2-to-Fuel approach lies in a proprietary multi-step biocatalytic process. Instead of using expensive inorganic catalysts, such as zinc, gold or zeolite, with traditional high energy catalytic chemical processes, our process uses inexpensive, renewable biomolecules to catalyze certain chemical reactions required to transform CO2 into basic hydrocarbon building blocks. Of greatest significance, our process occurs at low temperature and low pressure, thereby requiring far less energy than other approaches.”
The graphic illustrates the step-by-step process. The webpage goes on to say, “The biocatalysts employed in each step of the process serve to create intermediate hydrogen and carbon compounds that can be acted on by the next step with less energy. At the end of the process, these compounds are assembled into basic hydrocarbons – such as C1 (one carbon atom fuel – e.g. methane), C2 (two carbon atom fuel – e.g. ethane) and C3 (three carbon atom fuel – e.g. propane).”
Publicly traded Carbon Sciences finally explained where the hydrogen is coming from in last week’s comment by Dr Aslam who said, “Unlike other CO2 to fuel approaches, Carbon Sciences’ technology does not use molecular hydrogen (H2) because the creation and reaction of H2 is very energy intensive. Rather, the company’s approach is based on a low energy biocatalytic hydrolysis process where water molecules (H2O) are split into hydrogen atoms (H) and hydroxide ions (OH) using a biocatalyst. The hydrogen atoms (H) are immediately used in the production of hydrocarbons and the free electrons in OH are used to power the various biocatalytic processes.”
What that means is they’re going to use some water as a portion of the fuel and energy input. The technology “is based on natural organic chemistry processes that occur in all living organisms where carbon atoms, extracted from CO2, and hydrogen atoms extracted from H2O, are combined to create hydrocarbon molecules using biocatalysts and small amounts of energy. Our innovative technology allows this process to occur on a very large industrial scale through advance nano-engineering of the biocatalysts and highly efficient process design,” concluded Dr. Aslam.
All this leaves some unanswered questions – what will be the energy inputs other than the hydroxide, just how much water is needed, what are the attributes of the biocatalysts in manufacturing, costs and other relevant information, and the unexplored issues of risks?
Whatever those answers are, one thing is becoming quite clear. This is an example of biology and chemistry intersecting the technologies so making a serious impact on energy and fuel production. The new words, biocatalytic and biocatalysis are something to stay up on and give serious though to for venture investing, research and careers.
Should all the suggested technology be viable in both operation and economics all emitters of CO2 in volumes will have a new revenue source. Keep in mind the big question, the recovery of coal oxidized CO2 and natural gas oxidized CO2 fully reformed into fuels would have a huge impact on the total fuel supply and oil prices, so just how much fuel measured in say one precursor product such as propane displace in crude oil?” An answer like one ton of coal yields so many watts, and then become so much fuel product would do.
It’s going to be a very big number worldwide if all the fixed plant emitters CO2 could be reused once or more as liquid fuels. This is definitely something important to watch.
Comments
10 Comments so far



[…] … Industrial Park near Maryborough (remember that one?) or the joint animal refuge / pound? We keep hearing a common theme – council has no money for new projects – but what about old projects? If amalgamation cost the region so much how is it that council has not sought assistance from the State … Fuel From Thin Air […]
[…] … and natural or man-made disasters.” While the Army stresses the updates described in AR 500-3 relate to chemical, biological, nuclear attacks, “natural disasters” and “technical or man-made disasters or accidents,” current Army doctrine is also heavily weighted towards contingency planning for “civil … Fuel From Thin Air […]
[…] national renewable energy lab Fuel From Thin Air […]
[…] industrial lab design Fuel From Thin Air […]
[…] … the next generation of low carbon cars and today we are delivering on our promises. Work will continue next year when we produce our low carbon industrial strategy.” Lord Drayson, Minister of State for Science and Innovation, added: “The technologies for low carbon vehicles are developing fast … Fuel From Thin Air […]
[…] … difference between a naturally occurring or derived chemical, they’d just as soon take the brand they’re familiar with, and can get at the corner drugstore. As far as consumers are concerned, there may be little difference between such chemicals as chlorine dioxide (produced via the MMS protocol … Fuel From Thin Air […]
[…] Vote Fuel From Thin Air […]
ISO is a non-governmental organization established in 1947 in Geneva, Switzerland. Today, ISO has more than one hundred member countries. The mission of ISO is to promote the development of standardization and related activities in the global marketpla…
This post makes a lot of sense !
Awesome post. I so good to see someone taking the time to share this information