Mar
30
UCLA Researchers Use Electricity and CO2 to Make Butanol
March 30, 2012 | 7 Comments
The study paper is published today, March 30 2012, in the journal Science. The study explains how James Liao, UCLA’s Ralph M. Parsons Foundation Chair in Chemical Engineering, and his team use a method for storing electrical energy as chemical energy in higher alcohols, which then can be used as liquid transportation fuels.
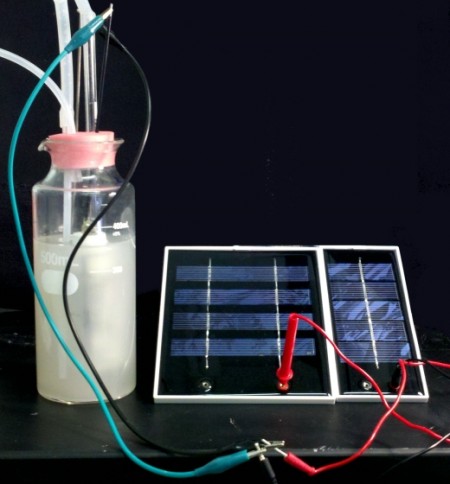
Liao and his team genetically engineered a lithoautotrophic microorganism known as Ralstonia eutropha H16 to produce isobutanol and 3-methyl-1-butanol in an electro-bioreactor using carbon dioxide as the sole carbon source and electricity as the sole energy input.
For background, photosynthesis is the process of converting light energy to chemical energy and storing it in the bonds of sugar. There are two parts to photosynthesis, a light reaction and a dark reaction. The light reaction converts light energy to chemical energy and must take place in the light. The dark reaction, which converts CO2 to sugar, doesn’t directly need light to occur.
Liao explains, “We’ve been able to separate the light reaction from the dark reaction and instead of using biological photosynthesis, we are using solar panels to convert the sunlight to electrical energy, then to a chemical intermediate, and using that to power carbon dioxide fixation to produce the fuel. This method could be more efficient than the biological system.”
Continuing, Liao said that with biological systems, the plants in use require large areas of agricultural land. However, because Liao’s method does not require the light and dark reactions to take place together, solar panels, for example, can be built in the desert or on rooftops. It’s becoming obvious that any electrical source would do.
Theoretically, the hydrogen generated by solar electricity can drive CO2 conversion in lithoautotrophic microorganisms engineered to synthesize high-energy dense liquid fuels. But the low solubility, low mass-transfer rate and the safety issues surrounding free hydrogen gas limit the efficiency and scalability of such processes. Instead Liao’s team found formic acid to be a favorable substitute and efficient energy carrier.
Liao goes on, “Instead of using hydrogen, we use formic acid as the intermediary,” said Liao and then describes the process, ”We use electricity to generate formic acid and then use the formic acid to power the CO2 fixation in bacteria in the dark to produce isobutanol and higher alcohols.”
With the process worked out the team now believes the electrochemical formate production and the biological CO2 fixation and higher alcohol synthesis open up the possibility of electricity-driven bioconversion of CO2 to a variety of chemicals. In addition, the transformation of formate into liquid fuel will also play an important role in the biomass refinery process.
Liao winds up the press release saying, “We’ve demonstrated the principle, and now we think we can scale up. That’s our next step.”
Perhaps then the bedeviling questions can be answered. How much electricity per unit of formic acid, how much formic acid and CO2 to what price of microorganisms, and what does a gallon cost? The press release is very vague.
Yet one can fully understand the “Eurkea!” feeling and the rush to publish. If the scale numbers work out well, the stampede to the researchers door will be quite something.
Liao points out an important point, “The current way to store electricity is with lithium ion batteries, in which the density is low, but when you store it in liquid fuel, the density could actually be very high. In addition, we have the potential to use electricity as transportation fuel without needing to change current infrastructure.”
This is an astonishing prospect. The sources for CO2 are a long list. Coal fired power plants alone generate an enormous store of CO2, relatively concentrated and not so hard to source. Using coal twice would an impressive accomplishment. Displacing some oil production would be a bonus. Depending on the state of the CO2 concentration crude oil could be a nearly obsolete product.
If the concentration required were low enough perhaps atmosphere alone would do as a source – an expectation quite hopeful, to say the least.
Liao has cracked into a huge potential opportunity. How the first step numbers come in will be interesting, but when the process concept shows its details Liao and his team may be credited for an entire new industry.
This news makes an important point – for any nation with any sense, very low cost electricity is going to be an economy supporting bedrock and expensive electricity a millstone to dragging an economy down.
The folks at UCLA must be very proud today – as we are of them.
Comments
7 Comments so far


I agree. Coal fired power plants would seem to be the obvious place to install this process – turning CO2 into fuel.
Let’s just hope the current regime in DC does not shut down all the coal plants before this process is refined.
Looking for a large and inexpensive source of concentrated and cheap carbonate?
Limestone rock.
Quicklime is the byproduct.
JP Straley
Energy required to reconvert CO2 to fuels is much more than the calorific value of the fuel produced. To make it feasible, one of the two choices is necessary.
1. Using free energy like sunlight for photosynthesis. Use of biomass can be a worthwhile input.
2. Non-carbon scalable energy like nuclear heat.
Yes this is the way for the future of renewable source of fuel, it’s double impact to our world it cleans CO2 emissions and alternative fuel source without impacting it like fracking in pacific ocean, not good.
A concreat plant may be a good location also when they burn lime you get a lot of CO2
Quite agree with you. A concreat plant may be a good location also when they burn lime you get a lot of CO2. Thanks for sharing.
Quite agree with you. A concreat plant may be a good location also when they burn lime you get a lot of CO2. Thanks for sharing.