Jul
21
Solar Hydrogen Fuel Production Cell Gets a 10 Fold Boost
July 21, 2015 | 1 Comment
Eindhoven University of Technology (TU/e) researchers have developed a very promising prototype of a new hydrogen producing solar cell. Using specially prepped gallium phosphide enables their solar cell to produce hydrogen gas for fuel from liquid water.
The Eindhoven group’s new design processes the gallium phosphide (GaP) in the form of very small nanowires. The new design helps to boost the yield by a factor of ten using ten thousand times less precious material.
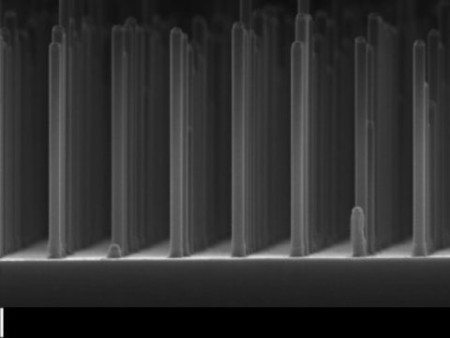
Gallium Phosphide Nanowire Array Produces Hydrogen. An array of nanowires gallium phosphide made with an electron microscope. Image Credit: Eindhoven University of Technology. Click image for the largest view.
GaP has good electrical properties but the drawback is that it cannot easily absorb light as large flat surface when used in GaP solar cells. The researchers have overcome this problem by making a grid of very small GaP nanowires, measuring five hundred nanometers (a millionth of a millimeter) long and ninety nanometers thick. This adaptation immediately boosted the yield of hydrogen by a factor of ten to 2.9 percent, a record for GaP cells. This remains some way off the fifteen percent achieved by silicon cells coupled to a battery.
For now to connect an existing silicon solar cell to a battery that splits the water may well be an efficient solution, but it is a very expensive solution. So many researchers are targeting their searches at semiconductor materials that are able to both convert sunlight into an electrical charge and split the water, all in one – a kind of ‘solar fuel panel’. The researchers at TU/e and FOM see their dream candidate in the gallium phosphide, a compound of gallium and phosphide. The GaP is also serving as the basis for specific colored LEDs.
Solar direct to a fuel is quite an objective. When electricity produced by a solar cell can be used to set off chemical reactions and generate a fuel, one can speak of solar fuels. Once the thresholds of practicality are reached there would be a hugely promising replacement for fossil fuels.
The possibility for now is to split liquid water using the solar generated electricity to drive electrolysis. That produces hydrogen gas and oxygen that can be used as a clean fuel in the chemical industry or directly recombusted in a chemical reaction to drive engines or recombined in fuel cells to produce electricity and used in cars for example.
According to research leader and TU/e professor Erik Bakkers, it’s not simply about the yield – where there is still a lot of scope for improvement. He points out, “For the nanowires we needed ten thousand times less precious GaP material than in cells with a flat surface. That makes these kinds of cells potentially a great deal cheaper. In addition, GaP is also able to extract oxygen from the water – so you then actually have a fuel cell in which you can temporarily store your solar energy. In short, for a solar fuels future we cannot ignore gallium phosphide any longer.”
The TU/e group is off to a great start. As efficiency climbs they’re eventually going to hit the hydrogen storage problem that haunts the entire hydrogen effort. But lets hope that doesn’t slow or stop them – more demand will incite more research and there is a storage solution out there somewhere in innovation.
Comments
1 Comment so far


I feel like this is another Good News/Bad News joke.
Good News: We have developed a hydrogen cell that is 10 times more efficient than any previously known.
Bad News: It’s ten microns across and costs $20,000.