Jul
1
Does Hydrogenation of CO2 Have a Future?
July 1, 2009 | 10 Comments
The US Naval Research Laboratory and the Center for Applied Energy Research at the University of Kentucky are investigating the hydrogenation of CO2 using a conventional Fischer-Tropsch cobalt catalyst for the production of valuable hydrocarbon materials. Using a straight dose of CO2 the researchers have managed to use catalysts to convert the gas and water vapor back into basic fuel products. Monday we had a look at research yielding a much more efficient CO2 collector. Now hydrogenation of CO2 is getting a little more attention.
The focus of this work is attempting to improve the production distribution toward the higher chain hydrocarbons and increase conversion rates using conventional Fischer-Tropsch catalysts. Backed by the U.S. Department of Defense, the motive is clear, the Department of Defense is the single largest buyer and consumer of fuel at 12.6 million barrels per day. That’s a big fuel user.
The research has been reported online June 25 in the ACS journal Energy & Fuels. Comparatively speaking, little research has been performed applying CO2 as the carbon source in synthetic fuel production, which as a blanket statement might be true, but activity in hydrogenation is ongoing. CO2 while a notability stable chemical, is thought to have too high of an energy barrier for polymerization, even in the presence of a catalyst. Gathering the CO2 to start and the processing of reforming each have energy costs to manage.
The researchers conducted the CO2 hydrogenation reactions in a one-liter three-phase slurry continuously stirred tank reactor. The team measured the ability to direct product distribution as a function of different feed gas ratios of H2 and CO2 (3:1, 2:1, and 1:1) as well as operating pressures ranging from 450 to 150 psig. Under all conditions investigated, methane remains the primary product, with concentrations ranging from 97.6% of the product to 93.1%. Higher concentrations of C2-C4 the range of ethane to butane hydrocarbons were found at the 1:1 ratio. The researchers also found that the portion of longer chain hydrocarbons, the hydrocarbons above methane increases with increasing time on stream, irrespective of the H2/CO2 ratio. That makes sense.
In the paper the team suggests that deactivation of the methane-forming active sites on the catalyst with increasing time on steam may play a role in the product distribution shift toward C2-C4 HC. Time on steam with the team’s catalysts seems to affect the product yield irrespective of the feed gas ratios. We can see that the energy input for this process is going to be the process heat to make steam.
Today it’s quite desirable to come out with higher carbon atom hydrocarbons. Methane or methanol fuels for fuel cells remains experimental although they would be great battery or capacitor rechargers across a wide range of products.
So the team’s effort is driving to longer carbon chains leads to directions such as exploring the hypothesis that the change in the feed gas ratio leads to a lowering of the catalyst’s methanation ability of CO2 in favor of chain growth, with two different active sites for methane and C2-C4 products present on the surface of the catalyst.
Steam drive is just one path. As Al Fin pointed out Monday in his comment that biology has been at this for billions of years. Another is sunlight drive.
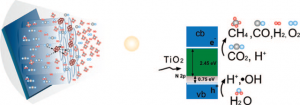
Researchers at Penn State are developing a method for the more efficient solar conversion of carbon dioxide and water vapor to methane and other hydrocarbons using nitrogen-doped titania nanotube arrays. These arrays feature a wall thickness low enough to facilitate effective carrier transfer to the adsorbing species, and are surface-loaded with nanodimensional islands of co-catalysts platinum (Pt) and/or copper (Cu).
The research yield has this rate of CO2 to hydrocarbon production obtained with outdoor sunlight up to at least 20 times higher than previous published reports. The previous work was done with UV illumination under laboratory conditions.
The Penn State photocatalytic methane-forming reaction requires eight photons, with additional photons required for other hydrocarbons. From the paper published in the January 27 issue of the ACS journal Nano Letters its said, “…we sought to enhance photocatalytic carbon dioxide conversion rates by using the following strategies: (i) employ high surface area titania nanotube arrays, with a wall thickness low enough to facilitate efficient transfer of photogenerated charge carriers to the surface species; (ii) modify the titania band gap to absorb and utilize the visible portion of the solar spectrum where the bulk of the solar energy lies; (iii) distribute cocatalyst nanoparticles on the nanotube array surface to adsorb the reactants and help the redox process.”
The gas sample analysis of the reaction products showed methane in high proportion, while ethane, propane, butane, pentane, and hexane as well as olefins and branched paraffins were also found in low concentrations. This is an interesting result as pentane and hexane are near gasoline carbon molecules. Paraffins are excellent bases for lubricants.
The Penn State team offers some interesting process conceptualization on the chemical energy during a conversion, “…a likely process in the photocatalytic reduction of CO2 by Cu or Pt loaded samples is the reduction of CO2 via the reaction CO2 + 2e- → CO + ½O2, which involves a free energy change of about 257 kJ/mol (1.33 eV per electron). The CO, thus formed, would react with atomic hydrogen to form hydrocarbons . . . Further rigorous studies are required to verify the validity of this hypothesis and understand the role of OH radicals and O2 in the possible back reactions.”
While this field is still small, the possibilities are incredible. Much more intelligence could be applied here. The connecting technologies are maturing as well. The Penn State team said it well, “We hope this work opens new avenues for carbon recycling using renewable sources.”
Indeed. There is a future for CO2 hydrogenation. A Hat Tip to Matt, you can read his thoughts in comments, for the prompt to write this post.
Comments
10 Comments so far


Useful blog website, keep me personally through searching it, I am seriously interested to find out another recommendation of it.
Since hydrogenation of CO2 is one way of actually reducing redundant CO2 from atmosphere, it certainly has future; especially when target is to yield synthetic hydrocarbon fuel products it is definitively worthwhile to be pursued. The trouble with fossil fuel is that it does not only produce CO2 upon combustion but also extra H2O in atmosphere,resulting eventually in steady rise of the sea level jointly with the melting of pole-ice. Synthetic HC fuel as opposed to fossil HC does not produce extra water on combustion in following scenario:
2H2O -> 2H2 + O2 (electrolysis)
2CO2 + 2H2 -> 2CO + 2H2O (catalysis)
nCO + mH2 -> HC + zH2O (Fischer-Tropsch)
The gist of the research is to seek for a most suitable catalyst for the reaction to be viable economically.
Cheers for this posting, guys, retain up the fantastic operate…
Oooh, you’re such an inspiration. I love this blog!
Awesome post. I so good to see someone taking the time to share this information
I was just having a conversation over this I am glad I came across this it cleared some of the questions I had.
Intriguing post. I have been searching for some good resources for solar panels and discovered your blog. Planning to bookmark this one!
Good! Thank you! I always wanted to write in my site something like that. Can I take part of your post to my blog?
Hello, this is my first time i visit here. I found so many interesting in your blog especially on how to determine the topic. keep up the good work.
Good article, but does anybody know what kind of catalysts were used for carbon dioxide hydrogenation in industrial use (especially for methane formation) and how effective is that catalysts (CO2 conversion, selectivity and activity) ? I will be greatfull for any information 🙂