Jun
15
Battery Research Prospects Grow
June 15, 2009 | 9 Comments
The basic idea flowing through battery research is to get power reacting metals with oxygen from air. It’s commonly done now, for sale wherever zinc-air hearing aid batteries are sold. But lithium, now famed for its batteries in cell phones and laptops and carbon in solid form, plus zinc, aluminum, and magnesium are under study using air.
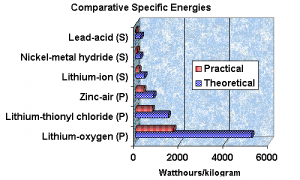
The potential, when the barriers and bugs are worked out are astounding. See the graph following:
As you can see the specific energies are quite good, although still way short of say the energy of fuels such as gasoline. But these numbers have can have a dramatic effect on practicality of electric propulsion and grid storage. Note the difference from the common lead acid battery. Things are changing and the coming change could well be that the unattractive and impractical become quite desirable.
The graph is from Battery Digest who asked Dr. Arthur Dooley to provide an expert opinion, “Specific capacity: For lithium metal alone 13 kWh/kg.
For the lithium and air, theoretical, 11,100 Wh/kg, not including the weight of oxygen, and 5,200 Wh/kg including the weight of oxygen. This was checked by calculation and agrees with K.M. Abrahams publication ,JECS 1996.
For the Lithium air cell, practical, 3,700 Wh/kg, not including the weight of oxygen, and 1,700 Wh/kg with the weight of oxygen.
These numbers are predictions and are made with the presumption that 33% of the theoretical energy will be obtained. The battery industry typically obtains 25% to 50% of the theoretical energy (Handbook of Batteries). Metal air batteries are higher in the range. Zinc-air is about 44% (Handbook of Batteries, 3rd Ed. pg 1.12 and 1.16 table and fig).”
“We selected a conservative 33%. You may quote these numbers above and make any comments with them. The theoretical numbers are similar to the numbers in the ECS 2004 abstract. (The difference is due to mathematical rounding.)”
So, what is the upper limit of energy density? The question has not been, and may never be, completely answered because the realm of possible can be included. The answers include factors such as acceptable safety, a dependence on the application, the environment of battery use, the economic factors and the realization that the battery for one application may not be the best choice for another.
Today and for as long as the research doesn’t set a new technology route to the top, a lithium anode with an air cathode to supply the oxygen may result in the highest practical energy density possible in a metal-based battery which has an abundant air supply, environmental friendliness, and reasonable safety. The key is in the possible recharge of lithium air designs.
There are problems to solve. Lithium-air battery cannot be put into a standby mode, unlike standard alkaline batteries, which just sit there when not used – for years. Open a lith-air to the air and they degrade – as current designs do – for now. The next matter is the power density. Today, unlike the high current providers of chemistries such as lead-acid, current densities of lith-air can be as much as 1,000 times lower in order to extract the maximum amount of energy. That means even though the lith-air has a huge amount of energy stored, it comes out really slowly. Low current output may not be a problem if the need is tailored to the output, but don’t look at lith-air as a replacement car starting battery with the current technology.
Next is the temperature matter. The performance of lith-air varies by a factor of 5 over the -20º C to +40º C range. Warmer is better, but keeping batteries warm is an energy matter of its own.
Undeterred, IBM Research is beginning an ambitious project that it hopes will lead to the commercialization of batteries that store 10 times as much energy as todays within the next five years. The company is planning to partner with U.S. national labs to develop the promising, but in some quarters, controversial lith-air technology. IBM is pursuing the technology instead of lithium-ion batteries because it has the potential to reach high enough energy densities to change the transportation system.
Chandrasekhar Narayan, manager of science and technology at IBM’s Almaden Research Center, in San Jose says, “With all foreseeable developments, lithium-ion batteries are only going to get about two times better than they are today,” he says. “To really make an impact on transportation and on the grid, you need higher energy density than that.”
The big problem is water in the air. Jeff Dahn, a professor of materials science at Dalhousie University, in Nova Scotia says, “Where there’s air there’s moisture, and humidity is the death of lithium.” When lithium metal meets water, an explosive reaction ensues. These batteries will require protective membranes that exclude water but let in oxygen, and are stable over time. Problems number one & two.
Lithium-metal batteries stalled about 20 years ago when a Canadian company Moli Energy recalled its rechargeable lithium-metal batteries, which didn’t use air but a more traditional cathode. After one caught fire, the incident led to legal action, and the company declared bankruptcy. After that Sony brought to market the first rechargeable lithium-ion batteries, which were safer, and research on lithium-metal electrodes slowed nearly to a halt until the performance pressures pushed the limits resulting in some more lith-ion batteries catching fire. By then it was too late to stop the technology.
Narayan says that lithium-air batteries are inherently safer than previously developed lithium-metal batteries as well as today’s lithium-ion batteries because only one of the reactants is contained in the cell. “A lithium-air cell needs air from outside,” says Narayan. “You will never get a runaway reaction because air is limited.” Narayan points to two remaining major problems with lithium metal-air technology. First, the design of the cathode needs to be optimized so that the lithium oxide that forms when oxygen is pulled inside the battery won’t block the oxygen intake channels. Second, better catalysts are needed to drive the reverse reaction that recharges the battery. A cursory view is quite encouraging.
Today the leader seems to be PolyPlus Battery in Berkeley, California who has been working on lithium metal-air technology for about six years. Polyplus has approached the challenge of the Lithium metal electrode with a coating of a glass-ceramic membrane, sealing the Lithium from an aqueous catholyte. PolyPlus also takes the widest view, with a lithium-metal battery pulling in oxygen from salt water as well as air. The technology is based on proprietary encapsulation of the lithium metal so its water stable enabling the practical realization of unique galvanic couples such as lith-air and lith-water batteries. The theoretical specific energy of lithium metal/aqueous couplings is greater than 10,000 Wh/kg and the first commercial batteries are expected to exceed 1,000 Wh/l and Wh/kg, much less than the common potentials noted by Dr. Dooley above.
There’s getting to be a lot of interest in lithium air battery technology. But don’t count out the exotics of today. Carbon, that nasty stuff to environmentalists was an early battery chemistry and may well develop into something great. Zinc air battery technology, the basis for all the hope in lithium and others isn’t completely finished yet. Aluminum, and magnesium only under academic study for now have potentials that aren’t reliably measured yet. Lots more to come.
As each or any of these get closer we’re going to look in. For now its lithium with all the promise it offers. A few years will tell much, but the demand is sure to go up and researchers are targeting 500 miles for transport range with good expectations that the problems can be solved. It is getting interesting.
Comments
9 Comments so far


I liked the last part of this article which quickly glossed over the other competing technologies. I see this type of battery being a great backend for grid storage. T don’t think any one battery/capacitor solution will be usefull here.
I also didn’t see how this would ever be usefull for vehicle transportation with such low power densities.
Keep posting stuff like this i really like it
very good \o/
Your thing is indeed unique in comparison with all kinds of other people. We appreciate you publishing for those who have the means,Guess Let me just be this bookmarked.2
Awesome post. I so good to see someone taking the time to share this information
Good! Thank you! I always wanted to write in my site something like that. Can I take part of your post to my blog?
I’ve been checking your blog for a while now, seems like everyday I learn something new 🙂 Thanks
Hello, this is my first time i visit here. I found so many interesting in your blog especially on how to determine the topic. keep up the good work.
You are correct so we built a battery that converts metal into electrical energy for months at a time. The patents are in place. New type of battery tech never seen before. from a small co, in Wyoming. with a smaller budget than any of the pro labs Ha Ha says this small co. Proven and tested by D.O.E. and is the real deal..