Aug
29
A New Leading Process For CO2 to Methanol
August 29, 2008 | 23 Comments
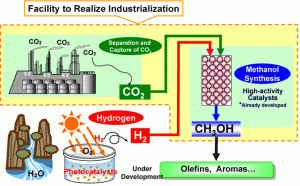
Mitsui Chemical Inc. of Japan has decided to begin construction of a pilot plant for continued development of producing methanol from industrial CO2 effluent and photocatalyst produced hydrogen. Due to build starting in October of 2008 with completion next February the plant is expected to go into use in March of 2010, the plant’s annual yield would work out to be the U.S. equivalent of over 33,000 gallons.
The construction will be at the company’s Osaka facilities where some 150 to 160 tons of CO2 can be obtained. The new plant is based on the cooperative work of Mitsui and the Research Institute of Innovative Technology for the Earth in Kyoto Japan. Mitsui has a nearly ten-year investment in the effort during the 1990s from which it is bringing a single unit photoelectrocatalyst hydrogen production technology. This process uses a highly efficient thin film, anatase titania photocatalyst that has a photon to current quantum efficiency of 60%. The yield of 220,000 pounds of methanol would need approximately 22% hydrogen by weight, some 48,400 pounds. Where the solar array will be installed isn’t discussed.
The CO2 side is from the Mitsui complex’s own process waste stream. The company makes the case that the cause of the research and investment is in mediation of CO2 emissions. It is a fully credible claim, yet the rise in petrochemical prices has to have a role in making the pilot investment worthwhile. The CO2 process is also based in results form the Research Institute’s “Chemical CO2 Immobilization Project.” Here Mitsui participated and took the research further with proprietary ultra high activity catalysts. This base research was in zinc oxide and copper catalysts that are upgraded to yield 44% efficiency for methane and 24% for ethylene from 82% of the carbon dioxide feed. This published rate does not deactivate the catalysts when operating in a pulsed bias.
The dollar investment is published at US$13.7 million or only $62.28 per annual gallon of capital cost. Without an operating expense it isn’t known how viable this venture might be, but the graph above notes that the output will be directed to valued added processes to yield things like olefins and aromatics.
The vague area is in the costs to sequester the CO2 and the use of the excess oxygen. It seems sensible that the unused 18% CO2 from the process would loop back in. The oxygen has value in O2 form and it’s a sure thing it won’t be vented as ozone.
Pilot plants are the first level scale up from the lab bench stage to check the real world practice and costs before trying an industrial sized plant. It’s quite interesting to see a number like less than $63.00 of capital costs even before the running expenses are factored in. What part of the capital costs would be the instrumentation that would not go forward to industrial sized builds isn’t known nor the other special one off costs that come from a first build.
What is illustrated is that in Japan at least, the sense that CO2 can be recycled back into the petrochemical stream is a viable idea that merits engineering and construction investments. It’s also noteworthy that they understand the importance of providing a source of hydrogen to maximize the product yield. The 82% CO2 used number is just outstanding. This is a much better concept than the US effort to somehow sequester and bury CO2. A few months ago the U.S. dropped a coal plant sequestration effort as it was simply too expensive. It is much smarter to make use of the value in the waste CO2 than try to make another kind of “landfill” for CO2. CO2 actually has value, as any green plant can tell you, and when processed up to a value added product it can stop being a topic of such contention.
Comments
23 Comments so far


Can anyone (like the writer of this piece) provide a few substantive technical references that might allow the serious reader to begin to separate the hype from the reality in this press release?
Let’s start with production of H2 using TiO2 catalysts. I believe the reported solar efficiencies (% of incident solar radiation that ends up in the H2) have ranged from about 0.1% to perhaps as high as 3% (though I can’t find the reference for the high end). For the best cases, fairly high platinum loadings are required, along with quite a bit of ethanol or methanol (some of which is consumed) in the water. Getting hydrogen this way has been extremely expensive.
Producing 100 kT MeOH annually is equivalent to an annual chemical energy output of about 2300 GJ, or an average output power of 74 kW. My guess is that the actual efficiency of conversion of H2 chemical energy to methanol chemical energy is about 25%. I base this guess on the fact that the conversion efficiency in the best methane to methanol plants (20,000 times larger) is about 65%.
The H2 can be obtained from electrical energy at 70% efficiency. To get the needed 420 kW of average PV power ($5.5/W-peak) would cost over $9M – for solar cells getting 18% efficiency.
It appears that the investment reported for the Mitsui plant ($14M) must refer only to the process for making methanol from CO2 and H2. It would be interesting to know how much they will spend to get the H2. I’m guessing it will cost at least $40M for a photocatalytic method. Since they mention a photo array, perhaps they’re not really using a photocatalytic method for generating most of the H2 they’ll be using.
The market value of the methanol produced will be about $50,000/year. Renewable methanol by this process cannot possibly compete with fossil-derived methanol for at least the next two decades. That’s the primary reason others have focused on higher value fuels from CO2, like ethanol and gasoline. See the WindFuels.com website. It appears to have much more potential to be market driven.
We are amidst depletion of resource of fossil fuels;Volumes of CO2 haunting & casting satanic shadows on the planet. Any Research on waste heat recovery,conversion of water through Solar power to Hydrogen combined with gainful usage of CO2 towards Methanol – even if the conversion efficiencies are discouraging at first instance,need &must be boosted, accelerated,pushed with ample good funding even globally. Successfully finding newer Energy resource shd be a top priority sector. Wish one sees the problem with good accent e.g wherefrom global average per capita consumption of 2,000 kwh pa wd come from at population of 10b from present 6b+ ?
Hello, I’m John M. Kocol, Founder & CEO of CO2toMethanol.com, an eQuarterback.com company. CO2toMethanol.com is the world’s first website to sell methanol made from carbon dioxide! CO2toMethanol.com was founded on April 18, 2009 which was a day after the historic “game changer” CO2-to-Methanol conversion breakthrough in Singapore at the Institute of Bioengineering and Nanotechnology (IBN).
Hello,I’m Oleksandr Perevertaylenko,Technical
University,Kharkiv,Ukraine.I’d joined to comments basing on information in Russian
media about 13/03/2010 of title:”Mitsui Chemicals Company will produce the material
for plastic production” in Russian.
The mentioned information is based on the
paper which is discussed.As I understood
Vice-President of MCCsaid that after sucsessful pilot plant tests the industrial
scale plant will be build during five years.
If so the technology of hydrogen obtaining
is realized.
As a specialist in heat exchange systems of
absorption gases purification I’m more interesting on information of CO2 capture from
flue gases in,ofcourse,permissible range.
With best regards
What do you think about converting CO2-to-Methanol? This new technology will help the U.S. become energy independent! Please visit: http://www.CO2toMethanol.com to learn more about this amazing breakthrough!
As I am informed now there are some developements devoted to use captured CO2 from flue gases as addition to methanol
Synthesis in existing methanol plants.
But direct conversion of CO2 to methanol is
another thing.It consists of two basic stages:
CO2 capture and H2 obtainig.The 1st stage
technologies are known and needs only on
improving and enhancing. The H2 obtaining on
my opinion is the bottleneck of CO2 to methanol conversion for industrial scale plant.The debottlenecking of this stage will
be of great importance.By the way according
to Mitsui information the small plant for
methanol production to be put into use this
month.So it will be interesting to know
about the results of this industrial experiment.
I would like to exchange links with your site newenergyandfuel.com
Is this possible?
Great story, bookmarked the blog in interest to read more!
CO2toMethanol.com has achieved a major carbon dioxide to methanol breakthrough!
The formal post assited me very much! Bookmarked the blog, very excellent topics just about everywhere that I see here! I appreciate the info, thank you.
Informative summary, saved the website in hopes to read more!
this post is very usefull thx!
De Methanol Economie, of de PTCB’s…
Gal Luft en de methanol economie De Methanol economie zou een running topic moeten zijn omdat deze zeer goed realiseerbaar is. De noodzakelijke industriele technologie is er, de zon voor de productie worden gebruikt, en hoewel methanol als brandstof ni…
I REALLY liked your post and blog! It took me a minute bit to find your site…but I bookmarked it. Would you mind if I posted a link back to your post?
I’ve been checking your blog for a while now, seems like everyday I learn something new 🙂 Thanks
Sure what are your questions?
Nice post! You truly have a wonderful way of writing which I find captivating! I will definitely be bookmarking you and returning to your blog. In fact, your post reminded me about a strange thing that happened to me the other day. I’ll tell you about that later…
please help me out about the counties or places converting CO2 to Methanol on plant or commercial scale.
I have a small hydrogen generator but can purchase more Hydrogen and have on site 5000 pound of liquid Co2 per hour 8 hours a day that has formed into vapor available to capture in a existing duct work to convert to methanol.
Any suggestion how will be appreciated
559-217-5071
I think the whole world needs comprehensive solutions in renewable energy. So far not many people like me to ask if we are using the right engine to power our transport, electricity generation and many other “big items” energy consumption. If we don’t recognized the fact that our world is using the wrong engines in these big items applications, we are doom to civilization failure because we are wasting too much energy everyday.
I think to understand why internal combustion engine for cars has no more than 15% energy efficiency is just as important as to understand why steam turbines only has 31% efficiency (Why hydro turbine can achieve 90%+?).
Without understanding the failure, and find the way to make energy waste to minimum, there is no point to find “cheap alternative” energy. We only have 200 years of history of “mordern era”. We are now feeling the pain of high energy cost. It is important to act now to find future energy solutions. But before that comes, we must understand all the engines we use, in cars, in ships, in the air, in the space, in the electricity power plant (thermal) ALL fail to achieve high energy efficiency.
I believe I have found the reasons why ICE energy efficiency is too low. Please visit my website blog section.
Crankshaft is only part of problem, I think. To use turbines for electricity generation and jet engine are wrong.
Tough the irony is: most investment goes to renewable energy research, not into area such as engine designing.
Marvelous posting, thanks for the information. It will assist me in many ways.
really loved the way info is been shared here.
CO2 to methanol requires a lot of energy. There is a requirement to add some free or low cost energy. The best is to use photosynthesis and work from biomass. Plant starch is the existing source of ethanol and methanol and the next is to convert lignin rather than burning it and prevent rather than collect at substantial cost.