Mar
30
Way Found to Increase Fuel Cell Efficiency
March 30, 2021 | 1 Comment
A Pohang University of Science & Technology research team revealed the phase transition and metal ex-solution phenomena to increase the catalytic activity in fuel cells.
Fuel cells, which are attracting attention as an eco-friendly energy source, obtain electricity and heat simultaneously through the reverse reaction of water electrolysis.
So, the catalyst that enhances the reaction efficiency is directly connected to the performance of the fuel cell. The POSTECH-UNIST joint research team has taken a step closer to developing high-performance catalysts by uncovering the ex-solution and phase transition phenomena at the atomic level for the first time.
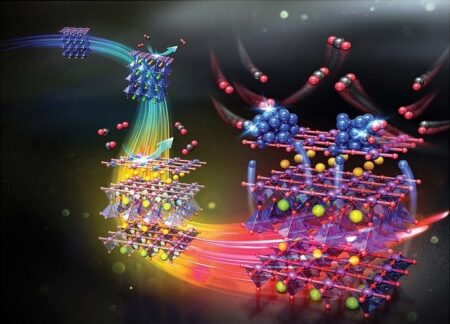
Visual insight into the phase transition and metal ez-solution phenomena from a simple perovskite to an enhanced catalyst. Image Credit: Pohang University of Science & Technology. Click image for the largest view.
A joint research team of Professor Jeong Woo Han and Ph.D. candidate Kyeounghak Kim of POSTECH’s Department of Chemical Engineering, and Professor Guntae Kim of UNIST have uncovered the mechanism by which PBMO – a catalyst used in fuel cells – is transformed from perovskite structure to layered structure with nanoparticles ex-solution to the surface, confirming its potential as an electrode and a chemical catalyst.
Catalysts are substances that enhance chemical reactions. PBMO (Pr0.5Ba0.5MnO3-δ), one of the catalysts for fuel cells, is known as a material that stably operates even when directly used as a hydrocarbon, not hydrogen. In particular, it exhibits high ionic conductivity as it changes to a layered structure under a reduction environment that loses oxygen. At the same time, the ex-solution phenomenon occurs in which the elements inside the metal oxide segregate to the surface.
This phenomenon occurs voluntarily under a reduction environment without any particular process. As the elements inside the material rise to the surface, the stability and performance of the fuel cell improve immensely. However, it was difficult to design the materials because the process through which these high-performance catalysts were formed was unknown.
Focusing on these features, the research team confirmed that the process goes through a progression of phase transition, particle ex-solution, and catalyst formation. This was proved using the first-principles calculation based on quantum mechanics and the in-situ XRD2 experiment that allows the observation of real-time crystal structural changes in materials. The researchers also confirmed that the oxidation catalyst developed this way displays up to four times better performance than the conventional catalysts, verifying that this study is applicable to various chemical catalysts.
Professor Jeong Woo Han who led the study explained, “We were able to accurately understand the materials in atomic units that were difficult to confirm in previous experiments, and successfully demonstrated it thus overcoming the limitations of existing research by accurately understanding materials in atomic units, which were difficult to confirm in existing experiments, and successfully demonstrating them. Since these support materials and nanocatalysts can be used for exhaust gas reduction, sensors, fuel cells, chemical catalysts, etc., active research in numerous fields is anticipated in the future.”
Complex molecules like perovskites seem to be turning up everywhere, even as catalysts to recombine hydrogen and water and may even puah out the expensive noble metals. If they do get to very high long lasting catalytic activity in fuel calls, without an environmental risk the fuel cell economy using hydrogen might finally get some legs.
Comments
1 Comment so far


Good! Interesting read, perhaps the best article iv’e browse today.