Feb
13
New Membrane To Help Make Methanol From CO2
February 13, 2020 | Leave a Comment
Rensselaer Polytechnic Institute chemical engineers have demonstrated how to convert CO2 to methanol more efficient by using a highly effective separation membrane they produced. Carbon dioxide, on one hand, is a greenhouse gas that is the unwanted byproduct of many industrial processes. In the other, methanol is a versatile and efficient chemical used as fuel in the production of countless products.
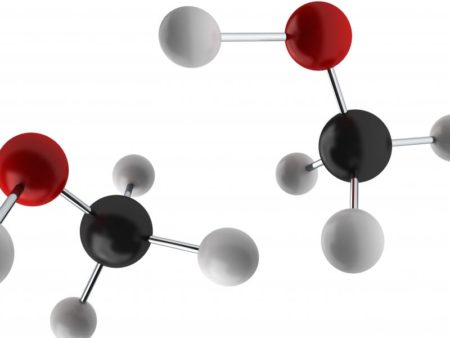
Methanol molecular structure where black is a carbon atom, white is hydrogen and red is oxygen. Image Credit: Rensselaer Polytechnic Institute, Click image for the largest view.
In a research paper published in Science, chemical engineers from Rensselaer Polytechnic Institute demonstrated how to make the conversion process from CO2 to methanol more efficient by using a highly effective separation membrane. This breakthrough, the researchers said, could improve a number of industry processes that depend on chemical reactions where water is a byproduct by recycling CO2.
For example, the chemical reaction responsible for the transformation of CO2 into methanol also produces water, which severely restricts the continued reaction. The Rensselaer team set out to find a way to filter out the water as the reaction is happening, without losing other essential gas molecules.
The researchers assembled a membrane made up of sodium ions and zeolite crystals that was able to carefully and quickly permeate water through small pores – known as water-conduction nanochannels – without losing gas molecules.
Miao Yu, an endowed chair professor of chemical and biological engineering and a member of the Center for Biotechnology and Interdisciplinary Studies (CBIS) at Rensselaer, who led this research said, “The sodium can actually regulate, or tune, gas permeation. It’s like the sodium ions are standing at the gate and only allow water to go through. When the inert gas comes in, the ions will block the gas.”
In the past, Yu said, this type of membrane was susceptible to defects that would allow other gas molecules to leak out. His team developed a new strategy to optimize the assembly of the crystals, which eliminated those defects.
When water was effectively removed from the process, Yu said, the team found that the chemical reaction was able to happen very quickly.
Huazheng Li, a postdoctoral researcher at Rensselaer and first author on the paper added, “When we can remove the water, the equilibrium shifts, which means more CO2 will be converted and more methanol will be produced.”
Deepak Vashishth, director of CBIS said, “This research is a prime example of the significant contributions Professor Yu and his team are making to address interdisciplinary challenges in the area of water, energy, and the environment. Development and deployment of such tailored membranes by Professor Yu’s group promise to be highly effective and practical.”
The team is now working to develop a scalable process and a startup company that would allow this membrane to be used commercially to produce high purity methanol.
Yu said this membrane could also be used to improve a number of other reactions.
“In industry there are so many reactions limited by water,” Yu said. “This is the only membrane that can work highly efficiently under the harsh reaction conditions.”
It will be interesting to see how this membrane can be made useful in other applications. Little thought is given by the masses to the processes in use that make modern life possible. These improvements, while seemingly of little consequence, often make massive improvements in products or great reductions in costs.

