Jan
23
Catalyst Found To Make Use Of Glycerol
January 23, 2020 | Leave a Comment
Tokyo Institute of Technology scientists have developed a cheap and efficient copper-based catalyst that can be used to convert glycerol to dihydroxyacetone (DHA). Glycerol, one of the main by-products of the biodiesel industry, has been a problem of excess supply and limited use. Additionally, this same process produces hydrogen molecules from water, and those molecules could be used as a clean type of fuel, further highlighting the impact of this research in terms of energy sustainability.
Although governments, academia, and organizations all around the world have been emphasizing the crisis concerning the use of fossil fuels for many years, the demand has constantly been on the increase. Researchers have fervently focused on finding alternative fuels that are cleaner and with the potential for sustainable production.
Hydrogen (H2) is a very attractive candidate as a replacement of fossil fuels because it can be produced from water (H2O) through hydrolysis, the splitting of water molecules. Another sustainable route is the synthesis of biodiesels, which are made using vegetable oils through a transformation process known as transesterification.
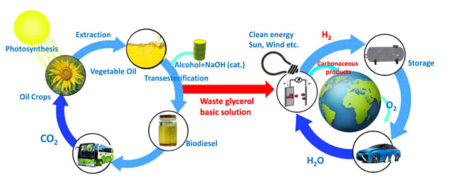
Sustainable biodiesel and hydrogen energy cycles. One of the main waste by-products of the biodiesel industry, glycerol, can be used as a raw material for the generation of valuable dihydroxyacetone and hydrogen, the latter of which can be used as 100% clean fuel. Image Credit: Tokyo Institute of Technology. Click image for the largest view.
However, biodiesel synthesis produces excessive amounts of glycerol (C3H8O3). It is estimated than the biodiesel industry in Europe alone produces a surplus of 1.4 million tons of glycerol, which cannot be sold to other industries. If glycerol could be used as a raw material to obtain more valuable chemicals, this would make the biodiesel industry more profitable, thus allowing governments, companies and consumers an alternative to fossil fuels.
Now, researchers from Tokyo Tech and Taiwan Tech recently found an efficient way to put this surplus glycerol to good use. While the electrochemical conversion of glycerol to other more valuable organic compounds, such as dihydroxyacetone (DHA), has been studied for years, existing approaches require the use of precious metals, namely platinum, gold, and silver. Because the use of these metals represents 95% of the overall cost of glycerol to DHA conversion, this research team focused on finding an affordable alternative.
In their study, they found that copper oxide (CuO), a cheap and abundant material, could be used as a catalyst to selectively convert glycerol into DHA even at mild reaction conditions. For this to happen, the pH (concentration of free hydrogen ions) in the solution of the electrochemical cell has to be at a specific value.
Through various microscopy techniques, the researchers analyzed the crystalline structure and composition of the CuO catalyst and tailored them to make it stable while also carefully inspecting the possible conversion pathways for glycerol in their system according to the solution’s pH. This allowed them to find appropriate reaction conditions that favored the production of DHA.
Professor Tomohiro Hayashi, lead researcher from Tokyo Tech noted, “We have not only discovered a new, earth-abundant catalyst for high-selectivity DHA conversion, but also demonstrate the possibility of giving new valuable life to a waste product of the biodiesel industry.”
As mentioned above, the electrochemical system proposed in this study not only produced DHA from glycerol on one end, but also H2 on the other through water splitting. This means that this approach could be used to address two current problems simultaneously. “Both the biodiesel and the hydrogen generation industries could benefit from our system, leading to a more sustainable world,” explained Prof. Hayashi.
The glycerol issue has met a huge breakthrough. Glycerol is now a plug limiting the progress of biodiesel market growth. Its mostly a case of getting rid of it at the lowest possible cost instead of using a process that produces a profit. This research may well be a sea change level of progress in biofuel production. There is a worldwide abundance of the major agricultural food crops and many areas and people that could be growing fuel crops.
Should this technology scale up to commercial scale it would be a world wide economic boon.

