Jun
12
Artificial Photosynthesis Makes CO2 Into Liquid Fuel
June 12, 2019 | 2 Comments
By converting carbon dioxide into more complex molecules like propane, green energy technology is now one step closer to using excess carbon dioxide to store solar energy – in the form of chemical bonds – for use when the sun is not shining and in times of peak demand.
Plants use sunlight to drive chemical reactions between water and CO2 to create and store solar energy in the form of energy-dense glucose. In their new study the researchers developed an artificial process that uses the same green light portion of the visible light spectrum used by plants during natural photosynthesis to convert CO2 and water into fuel, in conjunction with electron-rich gold nanoparticles that serve as a catalyst.
The new findings are published in the journal Nature Communications.
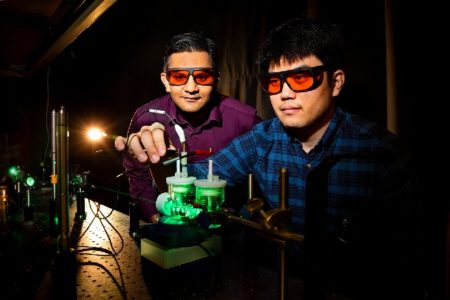
Jain, left, and Yu performing artificial photosynthesis experiments using green light. Photo by Fred Zwicky. Image Credit: UI at U-C. Click image for the largest view.
Prashant Jain, a chemistry professor and co-author of the study said, “The goal here is to produce complex, liquefiable hydrocarbons from excess CO2 and other sustainable resources such as sunlight. Liquid fuels are ideal because they are easier, safer and more economical to transport than gas and, because they are made from long-chain molecules, contain more bonds – meaning they pack energy more densely.”
In Jain’s lab, Sungju Yu, a postdoctoral researcher and first author of the study, uses metal catalysts to absorb green light and transfer electrons and protons needed for chemical reactions between CO2 and water – filling the role of the pigment chlorophyll in natural photosynthesis.
Gold nanoparticles work particularly well as a catalyst, Jain said, because their surfaces interact favorably with the CO2 molecules, are efficient at absorbing light and do not break down or degrade like other metals that can tarnish easily.
There are several ways in which the energy stored in bonds of the hydrocarbon fuel is freed. However, the easy conventional method of combustion ends up producing more CO2 – which is counterproductive to the notion of harvesting and storing solar energy in the first place, Jain noted.
“There are other, more unconventional potential uses from the hydrocarbons created from this process,” he said. “They could be used to power fuel cells for producing electrical current and voltage. There are labs across the world trying to figure out how the hydrocarbon-to-electricity conversion can be conducted efficiently.”
As exciting as the development of this CO2-to-liquid fuel may be for green energy technology, the researchers acknowledge that Jain’s artificial photosynthesis process is nowhere near as efficient as it is in plants.
“We need to learn how to tune the catalyst to increase the efficiency of the chemical reactions,” he said. “Then we can start the hard work of determining how to go about scaling up the process. And, like any unconventional energy technology, there will be many economic feasibility questions to be answered, as well.”
One day the world’s economy will be participating in the short term planetary carbon cycle. This work gets us closer. Carbon fuels are much less expensive for the consumer to capitalize for use – as most of the devices are already in place. Even if the only end product is propane, some pressure and it is liquid and has a history of lots of uses from fueling automobiles to heating homes.
As for efficiency, note plants on the whole are not amazingly efficient, and for all the hype about atmospheric CO2, its share of the atmosphere is pretty small. But its not so hard to imagine today’s oil companies harvesting propane and other materials from solar farms.
Comments
2 Comments so far


Short chain hydrocarbons are the ideal storage of energy. They could be used efficiently in fuel cells. They are easy tostoore and use ass brought out. In the article. More efficient production will be of use.
It would fit in nicely in a methanol economy as solar contribution as well as storage. All other parts including fuel cells should also be followed.