Mar
26
A Way Developed to Find New and Better Catalysts
March 26, 2019 | 1 Comment
University of Copenhagen researchers have developed a method that makes it easier to find better and cheaper catalysts. Catalysts that can ensure the creation and storage of bio source, solar and wind energy in fuels and chemicals will therefore play an increasingly important role.
The catalysts that are used today are often both expensive and less than optimally effective. Researchers at the University of Copenhagen and DTU have developed a method that makes it easier to find better and cheaper catalysts, with their results having recently been published in the journal Joule.
According to UN figures the world’s energy needs will increase two to three times over the next 30 years – as the world’s population goes from approximately 7.3 billion today to approx. 9.7 billion by 2050.
It won’t be enough to expand the capacity of bio sources, solar and wind energy as a substitute for fossil fuels. These sources satisfy the need for environmental sustainability, but they are unstable due to their reliance on unpredictable weather conditions.
A result of this instability is that catalysts and electrolysis have become increasingly important, in the hope that they are able to ensure a stable energy supply. In addition to this, catalysts are used for many things in the chemical industry; from the conversion of harmful exhaust gases from cars to the conversion of nitrogen from the atmosphere for fertilizers.
Professor Jan Rossmeisl at the Department of Chemistry for the University of Copenhagen, pointed out, “There is still a long way to go in the development of catalysts that can be used for e.g. fuel cells, storage of solar and wind energy and new environmentally friendly fuels. The catalysts that exist today are not good enough to ensure a green transition.”
With the aid of two PhD students, Jack K. Pedersen and Thomas A.A. Batchelor, he is looking for “the famous needle in the haystack” among a new generation of catalysts.
Professor Rossmeisl emphasized, “It is difficult to find the right alloy of metals for catalysts among infinitely many possibilities — despite today’s supercomputers. Finding the best alloys would take a lifetime. We use the so-called high-entropy alloys, which are random mixtures of many different elements, as a starting point and we have developed computer models based on machine learning. In this way, it becomes easier to sort the myriad of combinations of alloys and find those that can solve the problem of converting and storing solar and wind energy efficiently.”
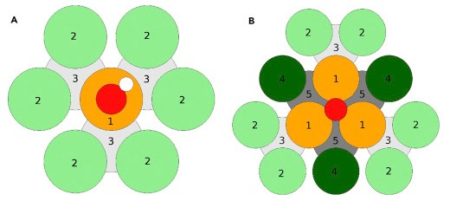
Parameterization of the Surface Configurations Using Nearest Neighbors. (A) *OH on-top binding. Each colored zone represents a set of 5 parameters. Orange (1): binding site. Light green (2): surface neighbors are coordinating once to the binding site. Light gray (3): subsurface neighbors are coordinating once to the binding site.
(B) *O fcc hollow site binding. Each colored zone represents a set of 5 parameters except zone 1, for which the set is 35 parameters. Zones 1–3 as in (A), and additionally: dark green (4): surface neighbors are coordinating twice to the binding site. Dark gray (5): subsurface neighbors coordinating twice to the binding site. Image Credit: University of Copenhagen. Use the link above to the study paper for a larger view and complete explanation.
The chemical industry uses catalysts for processes to run efficiently while remaining environmentally friendly, from the transformation of exhaust gases from cars to the production of fertilizers using nitrogen from the atmosphere. Among these chemical processes there are some that do not yet have effective catalysts, and these will require solutions in the near future. For example the conversion of carbon dioxide into useful substances to mitigate climate change, and the reaction between oxygen and hydrogen to form water for use in fuel cells. The role of a catalyst is to aid the conversion of chemical substances in a chemical reaction, and an effective catalyst can do this quickly and with small energy loss. It is a great challenge to predict which material will act as a good catalyst for a chemical reaction, and it is exactly this problem that the Copenhagen team proposed a solution for with a new class of materials, the so-called high-entropy alloys.
High-entropy alloys are a composed of a mixture of five or more metals, having only recently been used as catalysts. The team presented the first theoretical study of how to systematically benefit from high-entropy alloys to provide the best alloy candidate that can catalyze a desired chemical reaction.
What makes the high-entropy alloys different from other catalysts is that they have a surface with countless local configurations of different atoms giving rise to as many local chemical environments. Imagine a Rubik’s Cube: When it is solved, it consists of six faces each with its own color representing the pure metals. Mix the Rubik’s Cube and each face is now composed of many colors. On each face, the six colors can be arranged many different ways. The nine squares represent a local combination of six different metals on the surface of a high-entropy alloy. Some combinations of atoms on the surface will bind the reacting chemical substances weakly, while with others they will bind strongly. For those combinations of atoms where the bond strength is perfect the catalytic activity will be greatest, and these combinations will govern the overall catalytic activity.
By calculating the bond strength of the chemical substances for all configurations of atoms, the team can identify the best chemical environments and in what proportion the mixed metals are included at the atomic level. Here, however, the team encountered the problem that it would take a lifetime to calculate the bond strengths for all the combinations even with modern quantum mechanical methods. The team solved this problem by calculating the bond strengths of a randomly selected subset of the possible combinations and then used machine learning to calculate the bond strengths for the entire span of combinations in just a few seconds.
When the bond strengths of all local combinations of atoms on the surface are known the team is able to tune the ratio of the incorporated metals in order to promote the likelihood that the best bond strengths occur as frequently as possible. This optimal mixing ratio can be calculated and the outcomes are completely new, untested catalysts. The method thus gives a systematic way of proposing catalysts which only depends on which metals are included. The team used the method to suggest catalysts for the reaction between oxygen and hydrogen forming water but the application is very broad so they are currently working on several other chemical reactions, as well as improving the approximations and assumptions of the method so they can propose alloys that hopefully exceed the activity of present day catalysts.
Its reasonable to say that a future full of success is going to need lots more catalytic progress. This works looks like it can save a lot of time and drive lower reaction costs.
Comments
1 Comment so far


New energy contributes a lot to the world