Mar
6
Very Low Cost Hydrogen Extraction Catalyst Found
March 6, 2018 | Leave a Comment
Currently precious metals are the standard catalyst materials used for extracting hydrogen from water. The problem is these materials such as platinum, ruthenium and iridium – are too expensive. A team from Sweden’s KTH Royal Institute of Technology recently announced a breakthrough that could change the economics of a hydrogen economy.
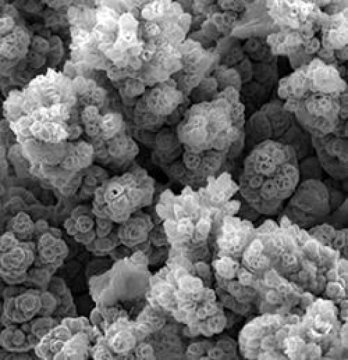
Electron microscope image depicts the new KTH water splitting alloy. Image Credit: KTH The Royal Institute of Technology. Click image for the largest view.
Led by Licheng Sun, professor of molecular electronics at KTH, the researchers concluded that precious metals can be replaced by a much cheaper combination of nickel, iron and copper (NiFeCu).
Researcher Peili Zhang said, “The new alloy can be used to split water into hydrogen. This catalyst becomes more efficient than the technologies available today, and significantly cheaper. This technology could enable a large-scale hydrogen production economy. Hydrogen can be used for example to reduce carbon dioxide from steel production or to produce diesel and aircraft fuel.”
This is not the first time a cheaper material has been proposed for water splitting, but the researchers argue that their solution is more effective than others.
They published their results recently in the scientific journal Nature Communication.
“The high catalytic performance of core-shell NiFeCu for water oxidation is attributed to the synergistic effect of Ni, Fe and Cu,” Zhang said.
Zhang explained that copper plays an interesting role in the preparation of the electrode. In an aqueous solution, surface copper dissolves and leave a very porous structure to enhance the electrochemically active surface area. “The porous oxide shell with its high electrochemically active surface area is responsible for the catalytic activity, while the metallic cores work as facile electron transport highways,” Zhang said.
Professor Sun has previously made progress in this field of research, including the construction of artificial photosynthesis (Nature Chem. 4/2012), and a catalyst based on nickel and vanadium (Nature Com. 7/2016). His and colleagues’ research was one of the reasons that US President Barack Obama went to KTH in 2013 during the second-ever state visit of an American president in Sweden.
The team has made a big claim here in the face of previous catalysts with great claims over the past few years. Part of the matter is the hydrogen economy is having trouble getting itself marketable in the face of petroleum’s history, ongoing success and drop in crude and natural gas prices.
One does hope the team has a home run here. Yet there remains a market problem of great significance. Hydrogen storage that is low cost, doesn’t leak, isn’t heavy, and has fairly quick load and unload times. Its a tall order.

