Oct
4
A Little Asphalt Makes a Better Lithium Ion Battery
October 4, 2017 | 3 Comments
Chemist James Tour’s lab at Rice University developed anodes comprising porous carbon made from asphalt that showed exceptional stability after more than 500 charge-discharge cycles. A high-current density of 20 milliamps per square centimeter demonstrated the material’s promise for use in rapid charge and discharge devices that require high-power density.
The finding is reported in the American Chemical Society journal ACS Nano.
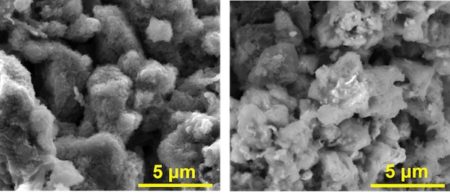
Scanning electron microscope images show an anode of asphalt, graphene nanoribbons and lithium at left and the same material without lithium at right. The material was developed at Rice University and shows promise for high-capacity lithium batteries that charge 20 times faster than commercial lithium-ion batteries. Image Credit: The Tour Group. Click image for the largest view.
Tour said, “The capacity of these batteries is enormous, but what is equally remarkable is that we can bring them from zero charge to full charge in five minutes, rather than the typical two hours or more needed with other batteries.”
The Tour lab previously used a derivative of asphalt – specifically, untreated gilsonite, the same type used for the battery – to capture greenhouse gases from natural gas. This time, the researchers mixed asphalt with conductive graphene nanoribbons and coated the composite with lithium metal through electrochemical deposition.
The lab combined the anode with a sulfurized-carbon cathode to make full batteries for testing. The batteries showed a high-power density of 1,322 watts per kilogram and high-energy density of 943 watt-hours per kilogram.
Testing revealed another significant benefit: The carbon mitigated the formation of lithium dendrites. These mossy deposits invade a battery’s electrolyte. If they extend far enough, they short-circuit the anode and cathode and can cause the battery to fail, catch fire or explode. But the asphalt-derived carbon prevents any dendrite formation.
An earlier project by the lab found that an anode of graphene and carbon nanotubes also prevented the formation of dendrites. Tour said the new composite is simpler.
Tour explained, “While the capacity between the former and this new battery is similar, approaching the theoretical limit of lithium metal, the new asphalt-derived carbon can take up more lithium metal per unit area, and it is much simpler and cheaper to make. There is no chemical vapor deposition step, no e-beam deposition step and no need to grow nanotubes from graphene, so manufacturing is greatly simplified.”
These are the most impressive lithium ion numbers one can ever recall. Tour is totally credible, but this work needs replication to entice industry to try for scaling up. Perhaps the Rice group will come up with a trade name for the technology like “Asphaltic Lithium” so we can look out for those batteries! Your humble writer definitely wants one for his cell phone.
Comments
3 Comments so far


The asphalt can make the better lithium battery. That technology should be applied broadly.
Good article! More people should know this technology.
Asphalt mixed with lithium-ion… awesome. More ‘defects’ and trial-and-error type additives are proving very interesting results. I like the organic direction such research is taking. This group is probably spurring their creativity through an axiomatic economic model, eg. use the cheapest materials like asphalt and see how they react.