Apr
13
Cleaner More Efficient Water Splitting For Hydrogen
April 13, 2017 | 1 Comment
Weizmann Institute of Science researchers have succeeded in almost fully suppressing the production of hydrogen peroxide during water splitting by controlling the spin of electrons in the reaction. Hydrogen peroxide is one of the main obstacles to making hydrogen production a reality as it is formed while water splitting proceeds. At high enough efficiency the use of solar energy to split water becomes more practical.
Hydrogen peroxide being formed affects both the efficiency of the reaction and the stability of the production process. Israeli and Dutch researchers from the Weizmann Institute of Science and Eindhoven University of Technology have succeeded in almost fully suppressing the production of hydrogen peroxide and erosion of the electrodes by controlling the spin of electrons in the reaction.
The research group’s findings have been published in the Journal of the American Chemical Society.
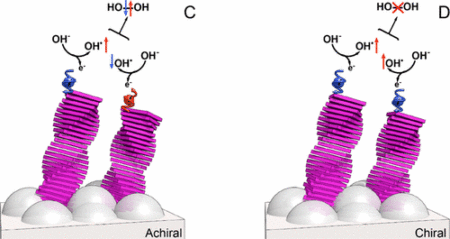
Supramolecular structures select spins only if they are chiral. Image Credit: Weizmann Institute of Science, Click image for the largest view.
The researcher’s goal is to produce hydrogen with photoelectrochemical (solar) cells, using light to split water. Unfortunately, the breaking apart of water molecules has been, up to now, relatively inefficient, and the hydrogen peroxide formed as a by-product corrodes some of the electrodes, thus further reducing the efficiency of the process.
The researchers, led by professors Ron Naaman of the Weizmann Institute of Science and Bert Meijer of Eindhoven University of Technology, are the first to have specifically investigated the role of the spin – the internal magnetic moment – of electrons involved in these basic, oxygen-based chemical reactions. They hypothesized that if both spins could be aligned, the formation of hydrogen peroxide would not occur, because the ground state of hydrogen peroxide needs two electrons with opposite spins. Oxygen, in contrast, is produced when the electrons have parallel spins.
The key to success was paint. The researchers covered one of the photoelectrochemical cell electrodes – the titanium-oxide anode – with organic paint containing chiral (molecules that are mirror images of each other), supramolecular structures of organic paint. These unique structures enabled the scientists to inject only electrons with their spins aligned in a certain direction into the chemical reaction.
This work was based on previous findings from Naaman’s lab group, demonstrating that the transmission of electrons through chiral molecules is selective, depending on the electrons’ spins. “The effect on water splitting exceeded our expectations,” said Naaman. “The formation of hydrogen peroxide was almost entirely suppressed. We also saw a significant increase in the cell’s current. And because chiral molecules are very common in nature, we expect this finding may have significance in many areas of research.”
The researchers are not yet able to say exactly how well this finding can improve the efficiency of hydrogen production. “Our goal was to be able to control the reaction and to understand what exactly was going on,” explained Meijer. “In some ways, this was a stroke of luck because the supramolecular structures had not originally been intended for this purpose. It goes to show how important supramolecular chemistry is as a fundamental field of research, and we’re very busy optimizing the process.”
Ah, the rush to publish – so we don’t know how much more efficiency this technology will gain. But what we will lose is very attractive. The home water splitting crowd knows about corroded electrodes and the nasty peroxide fluid left over. The most efficient electrodes are rare like platinum, or even switching out common stainless steel, being corroded away are simply not a practical efforts.
While the technology is new it does offer immense progress. Folks overlook hydrogen production backroom problems, and should this technology scale up the backroom matters will be far less of a problem.
Comments
1 Comment so far


Wondering if it would be possible to go the other way and encourage the
production of H2O2? Hydrogen Peroxide has lots of uses.