Sep
1
New Catalyst Makes Fuel Precursor from CO2 and Hydrogen
September 1, 2015 | Leave a Comment
University of Pittsburgh (UP) researchers have computationally derived a metal-free catalyst that captures converts and recycles the carbon dioxide into formic acid. The new metal-free catalyst that does the molecular recombining without the use of expensive or extreme conditions.
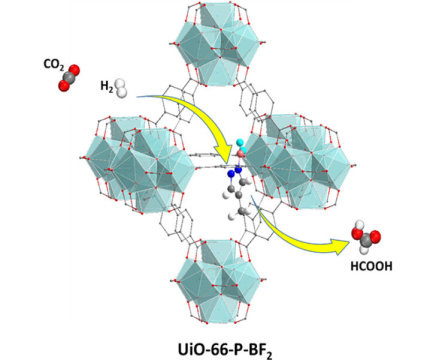
Carbon Dioxide Hydrogen Reforming Catalyst. A novel catalyst transforms carbon dioxide and hydrogen into formic acid (HCOOH) via a two-step (yellow arrows) reaction. This process combines the capture and conversion of carbon dioxide in a single chemical assembly (UiO-66-P-BF2). Image Credit: Ye and Johnson, University of Pittsburgh. © 2015 American Chemical Society. Click image for the largest view.
Turning carbon dioxide effluent into a more valuable chemical would reduce emissions while creating a revenue source. Finding ways to capture carbon dioxide molecules released into the atmosphere from coal-burning power plants is important for reducing the environmental impact as well as providing a pure stream of carbon dioxide to use for other purposes within industrial settings. This computationally derived catalyst able to capture and convert the carbon dioxide should provide design strategies for efficient, less expensive catalysts.
The research groups study paper has been published in the journal ACS Catalysis.
CO2 (carbon dioxide gas) released during the operation of coal-burning power plants to generate electricity is alleged to be one of the primary greenhouse gases. The release of CO2 presents a critical need to develop efficient and inexpensive catalysts to separate the greenhouse gas from other gases in exhaust streams before it enters the atmosphere.
Metal-organic frameworks (MOFs) are a form of physical adsorbents that have a cage-like structure built around atoms that include common metals or other molecules. They are excellent materials for storing carbon dioxide that suggests they could be useful for removing CO2 if outfitted with the proper catalyst. To reduce costs and make MOFs a viable industrial option, expensive metal catalysts must be replaced with a cheaper option.
A possible candidate for the UP team was using Lewis pair moieties that have both Lewis acid and Lewis base sites and are known to chemically bind CO2 while also causing hydrogen to dissociate. Using density functional theory the team designed a specific Lewis pair moiety that can be easily incorporated inside the MOF UiO-66 framework. Then, they used density functional theory calculations to show that the resulting material, UiO-66-PBF2, can dissociate hydrogen and add the resulting positive and negative hydrogen products to the carbon and oxygen atoms of CO2 molecules, respectively, to produce formic acid.
This process provides the potential to combine the CO2 capture and conversion steps in a single chemical assembly. The calculations indicate that the porous framework remains stable after both functionalization and chemisorption of CO2 and hydrogen.
In addition the research team found that activating hydrogen by dissociative adsorption leads to a much lower energy pathway for hydrogenating CO2, which suggests that this material may be a viable option for experimental testing. Future work will investigate other Lewis pair functional groups and how the addition of multiple binding sites per pore could allow for simultaneous activation of CO2 and hydrogen.
So far its all theoretical, but if actual constructed catalyst molecules perform as projected the field of catalyst applications will get a lot bigger and the possibilities for using and reusing chemicals will expand as well.

