Mar
25
New Understanding to Break Hydrogen Free of Biomass
March 25, 2015 | Leave a Comment
The A*STAR experimental analysis and computer simulations reveal new insights into the process by which ethanol produced from waste biomass can be converted into hydrogen in the presence of a catalyst. The new insights should aid the design of more efficient catalysts for hydrogen production.
Hydrogen gas is an environmentally friendly alternative to fossil fuels. Today using a process known as steam reforming, hydrogen is obtained by with steam to break up hydrocarbon compounds – most commonly, methane in natural gas. But ethanol produced by fermenting waste biomass is potentially a cleaner starting material for this process.
Despite having been extensively studied in recent years, steam reforming of ethanol is currently too inefficient to produce hydrogen on an industrial scale. This stems partly from the complexity of its reaction, which can yield a range of different products.
Jia Zhang of the A*STAR Institute of High Performance Computing in Singapore explains, “Our lack of understanding of the detailed reaction mechanism hinders further improvement of a catalyst for the reaction. The reaction was a black box before we started exploring it.”
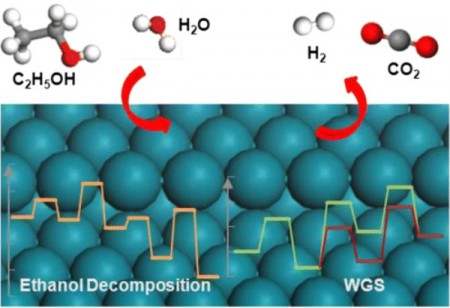
Now, Zhang and her co-workers have used experiments and computer simulations to probe how ethanol breaks down into hydrogen on rhodium catalysts supported on zirconia-based oxides. These nanosized catalysts had previously been shown to be highly active for this reaction.
The team used gas chromatography and mass spectrometry in real time to monitor the intermediate species that form as the reaction proceeds. The measurements revealed that the C2H4O species is an important intermediate. Of the two possible structures this species can adopt, acetaldehyde (CH3CHO) was identified as the most probable one by the team’s computer calculations. The calculations also showed that water plays an unexpectedly important role in controlling the reaction pathway.
Based on the new knowledge, the team proposed a mechanism for the reaction under their chosen conditions. Hydrogen is produced at most stages along the pathway, including the final step in which carbon monoxide reacts with water to produce hydrogen and carbon dioxide. The team’s calculations showed that the success of this final step is critical in determining the amount of hydrogen produced by steam reforming.
Zhang said, “Our theoretical simulations and experimental analysis provide important information on the reaction mechanism. This is a fundamental step forward in our understanding of the catalyst, which is the basis of catalyst design.”
The team’s ultimate goal is to design catalysts that can produce hydrogen more cheaply and efficiently than current catalysts.
The hydrogen for fuel effort is getting paved with lots of small but important steps that could well lead to low cost hydrogen. It surprises the uninitiated with all the possible sources from waste material, to biomass, splitting water and so on. It looks more promising all the time. Solve the production cost issue over enough sources and the two other problems of low cost fuel cells to use the hydrogen and safely store it without it escaping and the hydrogen economy could get real and big, fast.