Nov
11
Progress On the Ethanol to Hydrogen Fuel Path
November 11, 2014 | Leave a Comment
Hydrogen is a renewable resource with the potential to power everything from households to cars, but its use is currently limited by a lack of green and practical production methods. Then the storage issues arise and the combustion risks.
A*STAR researchers are helping to advance the development of hydrogen-powered cars by producing innovative materials that could make on-board hydrogen generators a reality. Their paper about the process has been published in Topics in Catalysis.
Today’s approach to generating hydrogen as a power source is done with steam reforming using primarily fossil fuels and electrolysis of water by applying an electric current with high energy input for the splitting of water into hydrogen and oxygen.
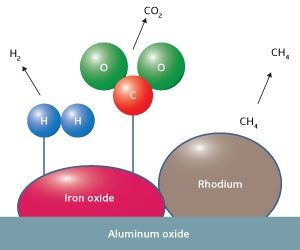
Schematic illustration of a rhodium-iron oxide catalyst on solid aluminium oxide for converting bioethanol into hydrogen gas at low temperature. Image Credit: The Agency for Science, Technology and Research. Click image for the largest view.
In contrast, the process of ethanol steam reforming (ESR) uses ethanol derived from renewable biomass to produce hydrogen and other products.
But the current ESR technology problems are that it requires high reaction temperatures to proceed and therefore a catalyst is needed to spur on the reaction. The other problem of ESR technology is that it often produces carbon monoxide as a byproduct, which is toxic and can also lead to poisoning of the hydrogen fuel cells that use the hydrogen to generate electricity.
The A*STAR team has developed an iron-promoted rhodium-based catalyst on a calcium-modified aluminum oxide support for the ESR process. This catalyst enables hydrogen to be generated more efficiently with less environmental damage as the reaction can occur at temperatures as low as 350º C (662º F) and produces almost no carbon monoxide as a byproduct.
The presence of iron oxide enables carbon monoxide to be converted into carbon dioxide and hydrogen via a reaction known as the water-gas shift reaction. Thus, the iron promotion effect on the rhodium-based catalyst is the key to removing carbon monoxide – something that is exceedingly difficult to achieve working with rhodium alone.
Additional benefits of the new ESR catalyst are the commercial advantages stemming from the catalyst being quite stable and having a long active lifetime. That means the catalyst will permit long cycle lengths, minimize the regeneration frequency and reduce the operational downtime for on-board steam reformers.
Chen explains that these factors are, “essential for maintaining profitable operations in reforming units. Similarly, a stable catalyst would reduce the operating cost for an on-board reformer.”
Chen noted that the catalyst will enable, “better operational flexibility in terms of economics and on-board reformer size (since carbon monoxide purification units can be removed).” That she said will, “make a significant impact in the design of efficient and simple on-board reactors.”
This research is promising for advancing the realization of small scale on-board reformers for portable and mobile power needs. While 660º is very hot, its closer to practical applications say in start and run all day kind of uses. It might not be the best choice for start, stop, short trip, urban kinds of driving.
ESR may not be the hydrogen economy folks dream come true; its utilization in the market would be closer to a closed loop carbon cycle of renewable fuel. The news should cheer up the ethanol produces getting another use for the fuel closer to market.

