Aug
6
A One Step & Efficient CO2 to Methanol Catalyst System
August 6, 2014 | 1 Comment
Brookhaven National Laboratory scientists have discovered a new catalytic system for converting carbon dioxide to methanol. The new system offers significantly higher activity than other catalysts now in use and the new system could make it easier to get normally unreactive CO2 to participate in these reactions.
Brookhaven chemist Jose Rodriguez, who led the research said, “Developing an effective catalyst for synthesizing methanol from CO2 could greatly expand the use of this abundant gas as an economical feedstock.” It’s even possible to imagine a future in which such catalysts help mitigate the accumulation of this greenhouse gas, by capturing CO2 emitted from methanol-powered combustion engines and fuel cells, and recycling it to synthesize new fuel.
But, Rodriguez emphasized that future, of course, will be determined by a variety of factors, including economics. “Our basic research studies are focused on the science, the discovery of how such catalysts work, and the use of this knowledge to improve their activity and selectivity,” he said.
Because CO2 is normally such a reluctant participant in chemical reactions, interacting weakly with most catalysts makes it also rather difficult to study. The Brookhaven studies required the use of newly developed in-situ imaging and chemical “fingerprinting” techniques. These techniques allowed the scientists to peer into the dynamic evolution of a variety of catalysts as they operated in real time. The scientists also used computational modeling at the University of Seville and the Barcelona Supercomputing Center to provide a molecular description of the methanol synthesis mechanism.
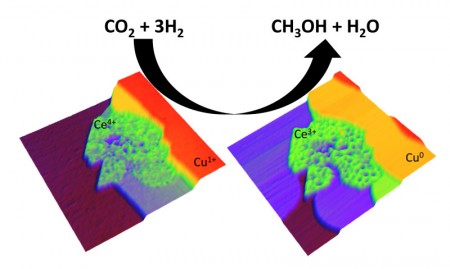
The team was particularly interested in exploring a catalyst composed of copper and ceria (cerium-oxide) nanoparticles, sometimes also mixed with titania. The scientists’ previous studies with such metal-oxide nanoparticle catalysts demonstrated their exceptional reactivity in a variety of reactions. In those studies, the interfaces of the two types of nanoparticles turned out to be critical to the reactivity of the catalysts, with highly reactive sites forming at regions where the two phases meet.
To explore the reactivity of such dual particle catalytic systems in converting CO2 to methanol, the scientists used spectroscopic techniques to investigate the interaction of CO2 with plain copper, plain cerium-oxide, and cerium-oxide/copper surfaces at a range of reaction temperatures and pressures. Chemical fingerprinting was combined with computational modeling to reveal the most probable progression of intermediates as the reaction from CO2 to methanol proceeded.
These studies revealed that the metal component of the catalysts alone could not carry out all the chemical steps necessary for the production of methanol. The most effective binding and activation of CO2 occurred at the interfaces between metal and oxide nanoparticles in the cerium-oxide/copper catalytic system.
Jesus Graciani, a chemist from the University of Seville and first author of the paper said, “The key active sites for the chemical transformations involved atoms from the metal [copper] and oxide [ceria or ceria/titania] phases.” The resulting catalyst converts CO2 to methanol more than a thousand times faster than plain copper particles, and almost 90 times faster than a common copper/zinc-oxide catalyst currently in industrial use.
The study illustrates the substantial benefits that can be obtained by properly tuning the properties of a metal-oxide interface in catalysts for methanol synthesis.
Brookhaven Lab Chemistry Department Chair Alex Harris said, “It is a very interesting step, and appears to create a new strategy for the design of highly active catalysts for the synthesis of alcohols and related molecules.”
At ninety times faster rates this work is sure to attract some industrial notice. While the operating conditions aren’t discussed, we do know that by hydrogenating CO2, the new catalyst set can transform the common combustion effluent gas into methanol, a desirable fuel and chemical raw material.
This is good work that offers another possible means to reuse the products from oxidizing fuels.
Comments
1 Comment so far


If this is true then we will have to reopen all those coal power plants because we need the CO2.
/s