Feb
5
Better Solar Cells From Understanding Charge Separation
February 5, 2014 | Leave a Comment
A new understanding of the basic science of charge separation offers new insight on making cheaper organic solar cells. The researchers suggest improved design rules for making more efficient solar cells in the future.
Today’s organic solar cells currently have a top efficiency of approximately 10 percent in the laboratory, much less than inorganic single crystal silicon based design. One of the challenges to realizing efficient organic cells lies in separating the strongly bound pairs made up of a negatively charged electron and its positively charged hole that result from light absorption, collectively referred to as an exciton. The electron and hole need to be separated in order to make an electrical current.
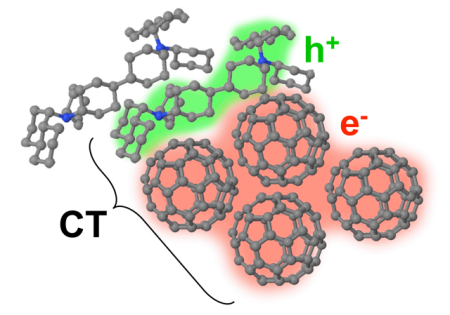
Nanocrystalline Fullerene Molecules in an Organic Solar Cell Heterojunction. Click image for more info.
The way this is done is by creating a heterojunction, which is two different organic semiconductors next to each other, one of which likes to give up an electron and the other which accepts the electron, thereby splitting the original exciton into an electron and hole residing on nearby molecules. A long-standing question in the field, however, is how the nearby electron and hole – still strongly attracted to each other at this stage – manage to separate completely in order to generate current with the efficiency observed in most solar cells.
Over the past few years, a new perspective has proposed that the high separation efficiency relies on a quantum effect – the electron or hole can exist in a wavelike state spread out over several nearby molecules at the same time. When the wave function of one of the carriers collapses at a location far enough away from its partner, the charges can separate more easily.
Giebink and colleagues’ work provide compelling new evidence to support this interpretation and identify nanocrystallinity of the common acceptor material made of C60 molecules (also known as fullerenes or buckyballs) as the key that allows this delocalization effect to take place.
Giebink explains this local crystalline order appears to be critical to efficient photocurrent generation in organic solar cells. “A common view in the community is that it takes a bunch of excess energy to break apart the exciton, which meant that there had to be a large energy level difference between the donor and acceptor materials. But that big energy offset reduces the voltage of the solar cell. Our work dispels this perceived tradeoff in light of the impact that wavefunction delocalization and local crystallinity have on the charge separation process. This result should help people design new molecules and optimize donor and acceptor morphologies that help increase solar cell voltage without sacrificing current,” he said.
The team used various luminescence and electroabsorption spectroscopic techniques together with X-ray diffraction to reach their conclusion. Their results, detailed in the paper titled “Delocalization and Dielectric Screening of Charge Transfer States In Organic Photovoltaic Cells,” will provide other groups with a better understanding of charge separation as they design and model more efficient organic solar cells.
This is a long way from a new manufacturing process. The organic solar cell has been stuck for years in achieving worthwhile efficiencies. Perhaps the team’s work will assist some innovators to design cells that can bring the costs and efficiencies up to more worthwhile levels.
It’s welcome news that we all hope can be integrated into design efforts soon.

