Sep
18
Exoelectrogenic Microbes Hooked Up to Make Power
September 18, 2013 | Leave a Comment
Stanford University engineers have devised a new way to generate electricity from sewage using naturally occurring “wired microbes” as mini power plants. The exoelectrogenic microbes produce electricity as they digest plant and animal waste.
The laboratory prototype is about the size of a D-cell battery and looks like a chemistry experiment, with two electrodes, one positive, the other negative, plunged into a bottle of wastewater. Inside the vial, attached to the negative electrode like barnacles to a ship’s hull, an unusual type of bacteria feast on particles of organic waste and produce electricity that is captured by the battery’s positive electrode.
Co-authors Yi Cui, a materials scientist, Craig Criddle, an environmental engineer, and Xing Xie, an interdisciplinary fellow, calls their invention a microbial battery in a paper published in the Proceedings of the National Academy of Sciences.
Scientists have long known of the existence of what they call exoelectrogenic microbes – organisms that evolved in airless environments and developed the ability to react with oxide minerals rather than breathe oxygen as we do to convert organic nutrients into biological fuel.
During the past dozen years or so, several research groups have tried various ways to use these microbes as bio-generators, but tapping this energy efficiently has proven challenging. What is new about the Stanford microbial battery is a simple yet efficient design that puts these exoelectrogenic bacteria to work.
Criddle, a professor in the department of civil and environmental engineering and a senior fellow at the Stanford Woods Institute for the Environment said, “We call it fishing for electrons.”
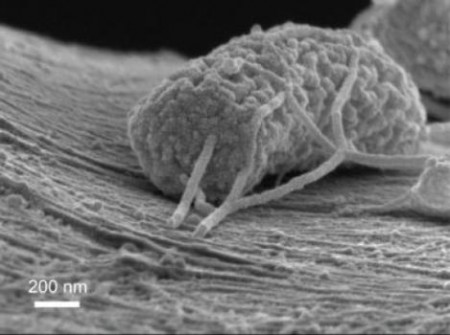
At the battery’s negative electrode, colonies of wired microbes cling to carbon filaments that serve as efficient electrical conductors. Using a scanning electron microscope, the Stanford team captured images of these microbes attaching milky tendrils to the carbon filaments.
“You can see that the microbes make nanowires to dump off their excess electrons,” Criddle said. To put the images into perspective, about 100 of these microbes could fit, side by side, in the width of a human hair.
As these microbes ingest organic matter and convert it into biological fuel, their excess electrons flow into the carbon filaments and across to the positive electrode, which is made of silver oxide, a material that attracts electrons.
The electrons flowing to the positive node gradually reduce the silver oxide to silver, storing the spare electrons in the process. According to Xie, after a day or so the positive electrode has absorbed a full load of electrons and has largely been converted into silver.
At that point the silver is removed from the battery and re-oxidized back to silver oxide. That releases the stored electrons to make power.
The Stanford engineers estimate that the microbial battery can extract about 30% of the potential energy locked in wastewater. That is roughly the same efficiency at which the best commercially available solar cells convert sunlight into electricity. It also looks competitive to simple combustion of the carbon elements in the waste.
Of course, there is far less energy potential in wastewater. Even so, the inventors say the microbial battery is worth pursuing because it could offset some of the electricity now use to treat wastewater. That use currently accounts for about three percent of the total electrical load in developed nations. Most of this electricity goes toward pumping air into wastewater at conventional treatment plants where ordinary bacteria use oxygen in the course of digestion, just like humans and other animals.
For the future the Stanford engineers say their biggest challenge will be finding a cheap but efficient material for the positive node.
Cui, an associate professor of materials science and engineering overlooks the prospects with, “We demonstrated the principle using silver oxide, but silver is too expensive for use at large scale. Though the search is underway for a more practical material, finding a substitute will take time.”
It idea looks pretty capital intensive and would have an ongoing maintenance expense. But the Stanford team is first with real power out, even though the little battery sized lab vial test rig doesn’t reveal the watts or volts that come from the silver reprocessing.
It’s a start, and a good one. More innovation will come, especially if that 30% energy recovery is a good number or estimated low. There is also the question of the remainder, the value in the undigested materials and its safety. The idea might be a breakthrough of another kind not yet thought about.