Jun
14
A New Way to Make Hydrogen For Fuel
June 14, 2013 | 4 Comments
Raymond Schaak, a professor of chemistry at Penn State University with his research team members have found that an important chemical reaction that generates hydrogen from water is effectively triggered – or catalyzed – by a nanoparticle composed of nickel and phosphorus, two inexpensive elements that are abundant on Earth.
The new discovery may well lead to cheaper hydrogen production technologies.
The results of the research have been published in the Journal of the American Chemical Society.
Schaak explained that the purpose of the nickel phosphide nanoparticle is to help produce hydrogen from water, which is a process that is important for many energy-production technologies, including fuel cells and solar cells.
“Water is an ideal fuel, because it is cheap and abundant, but we need to be able to extract hydrogen from it,” Schaak said. Hydrogen has a high energy density and is a great energy carrier, Schaak explained, but it requires energy to produce. To make its production practical, scientists have been hunting for a way to trigger the required chemical reactions with an inexpensive catalyst.
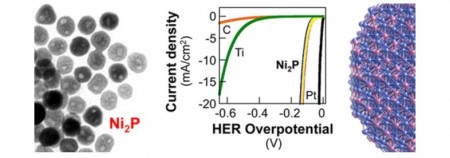
Nickel Phosphide Catalytic Nanoparticle Information. Click image for the largest view. More information at the study paper link above.
Schaak noted that this feat is accomplished very well by platinum but, because platinum is expensive and relatively rare, he and his team have been searching for alternative materials. “There were some predictions that nickel phosphide might be a good candidate, and we had already been working with nickel phosphide nanoparticles for several years,” Schaak said. “It turns out that nanoparticles of nickel phosphide are indeed active for producing hydrogen and are comparable to the best known alternatives to platinum.”
To create the nickel phosphide nanoparticles the team members began with metal salts that are commercially available. They then dissolved these salts in solvents, added other chemical ingredients, and heated the solution to allow the nanoparticles to form. The researchers were able create a nanoparticle that was quasi-spherical – not a perfect sphere, but spherical with many flat, exposed edges. “The small size of the nanoparticles creates a high surface area, and the exposed edges means that a large number of sites are available to catalyze the chemical reaction that produces hydrogen,” Schaak explained.
The next step took place at the California Institute of Technology where team members tested the nanoparticles’ performance in catalyzing the necessary chemical reactions. This segment of the research was led by Nathan S. Lewis, the George L. Argyros Professor of Chemistry at the California Institute of Technology. The researchers performed the tests by placing the nanoparticles onto a sheet of titanium foil and immersing that sheet in a solution of sulfuric acid. Next, the researchers applied a voltage and measured the current produced. They found that, not only were the chemical reactions happening as they had hoped, they also were happening with a high degree of efficacy.
“Nanoparticle technology has already started to open the door to cheaper and cleaner energy that is also efficient and useful,” Schaak said. “The goal now is to further improve the performance of these nanoparticles and to understand what makes them function the way they do. Also, our team members believe that our success with nickel phosphide can pave the way toward the discovery of other new catalysts that also are composed of Earth-abundant materials. Insights from this discovery may lead to even better catalysts in the future.”
The researchers working with Schaak and Lewis who contributed to this study include Eric J. Popczun, Carlos G. Read, Adam J. Biacchi, and Alex M. Wiltrout from Penn State; and James R. McKone from the California Institute of Technology.
The curious fact is the nanoparticles of nickel phosphide are hollow and faceted to expose a high density of the nickel phosphide surface, which had previously been predicted based on theory to be an active HER catalyst.
The press release and the abstract both leave us without an efficiency result. Avoiding platinum alone will make a huge difference, but both nickel phosphide and platinum will need a source of energy to perform the electrolysis.
Hydrogen could very well be a fuel for the future if the economy climate and political interference can lure out the lowest possible cost means to produce electric current to drive the reaction.
This team’s work is at such an early stage that many questions are due up for answering soon. We’d like to know about the products, is it mono or di hydrogen and oxygen products, the watts needed per unit and the proposed mechanics to separate the gases.
Looks good as far as they’ve gotten, let’s hope they get much further successfully.
Comments
4 Comments so far


I want to to thank you for this fantastic read!! I absolutely loved every
bit of it. I have got you book marked to check out new things you post…
I don’t even know how I stopped up here, but I assumed this post was once great.
I do not realize who you’re but certainly you are going to
a famous blogger if you are not already. Cheers!
Hydrocarbon fuel is the future. We just need everyone on board!
It’s genuinely very complicated in this full of activity
life to listen news on TV, thus I just use world wide web for that purpose,
and take the most recent news.