Mar
19
Build a Super Capacitor with a DVD Burner
March 19, 2012 | 1 Comment
The insightful and clever folks at UCLA have used a standard LightScribe DVD optical drive ($25+ and up at Newegg.com today) to produce a new type of capacitor electrodes that not only maintain high conductivity but also provide higher and more accessible surface area than conventional electrochemical capacitors (ECs) that use the typical activated carbon electrodes.
EC or electrochemical capacitor is a tech name for super or ultra capacitors. With more capacity these aren’t the ones seen inside the computer or TV. We’ve been watching closely as some firms like EEstor have attracted a lot of attention because the super or ultra capacitor could change the battery capacity needs of high demand applications like electric vehicles.
A real super or ultra capacitor that combines the power performance of capacitors with the high energy density of batteries would represent a significant advance in energy storage technology.
The UCLA team’s new electrodes are composed of an expanded network of graphene, a one-atom-thick layer of graphitic carbon that shows excellent mechanical and electrical properties as well as exceptionally high surface area.
Before we start, a Lightscribe DVD is one that can have an image burned on the label side by the laser in the DVD burner. That noted:
The process is based on coating a DVD disc with a film of graphite oxide that is then laser treated inside a LightScribe DVD drive to produce graphene electrodes. Typically, the performance of energy storage devices is evaluated by two main figures, the energy density and power density. Suppose we are using the device to run an electric car. The energy density tells us how far the car can go a single charge whereas the power density tells us how fast the car can go.
The UCLA devices made with Laser Scribed Graphene (LSG) electrodes exhibit ultrahigh energy density values in different electrolytes while maintaining the high power density and excellent cycle stability of ECs. Moreover, these ECs maintain excellent electrochemical attributes under high mechanical stress and thus hold promise for high power, flexible electronics.
The work comes out of the UCLA Department of Chemistry and Biochemistry, the Department of Materials Science and Engineering, and the California NanoSystems Institute. That very thin layer of electrode burned on the disk demonstrates a high-performance graphene-based electrochemical capacitor that maintains excellent electrochemical attributes under high mechanical stress. The team’s paper has been published in the journal Science.
Richard B. Kaner, professor of chemistry & materials science and engineering, points out the impressive expectation saying, “Our study demonstrates that our new graphene-based supercapacitors store as much charge as conventional batteries, but can be charged and discharged a hundred to a thousand times faster.” We’ll need weights and volumes soon to validate that.
Maher F. El-Kady, a graduate student in Kaner’s lab and the study lead author sums up the study saying, “Here, we present a strategy for the production of high-performance graphene-based ECs through a simple all solid-state approach that avoids the restacking of graphene sheets.”
The team fabricated LSG electrodes without the problems of activated carbon electrodes that have so far limited the performance of commercial ECs. First, The LightScribe laser causes the simultaneous reduction and exfoliation of graphite oxide and produces an open network of LSG with substantially higher and more accessible surface area. This results in a sizable charge storage capacity for the LSG supercapacitors. The open network structure of the electrodes helps minimize the diffusion path of electrolyte ions, which is crucial for charging the device. This can be accounted for by the easily accessible flat graphene sheets, whereas most of the surface area of activated carbon resides in very small pores that limit the diffusion of ions. This means that LSG supercapacitors have the ability to deliver ultrahigh power in a short period of time whereas activated carbon cannot.
That begs the question of how much more capacity could be gained by a laser cutting expressly for the maximum capacity. A common DVD burner is a stroke of genius for an experiment, but the potential must be considerable higher.
The graphene laid thin and flat is a mechanically robust and shows high conductivity (>1700 S/m) compared to activated carbons (10-100 S/m). This means that LSG electrodes can be directly used as supercapacitor electrodes without the need for binders or current collectors as is the case for conventional activated carbon ECs. Furthermore, these properties allow LSG to act as both the active material and current collector in the EC. The combination of both functions in a single layer leads to a simplified architecture and makes LSG supercapacitors cost-effective devices.
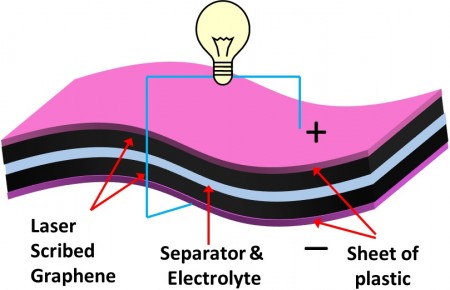
Today’s commercially available ECs consist of a separator sandwiched between two electrodes with liquid electrolyte that is either spirally wound and packaged into a cylindrical container or stacked into a button cell. Unfortunately, these device architectures not only suffer from possible harmful leakage of electrolytes, but their design makes it difficult to use them for practical flexible electronics.
The research team replaced the liquid electrolyte with a polymer gelled electrolyte that also acts as a separator, further reducing the device thickness and weight and simplifying the fabrication process as it does not require special packaging materials.
In order to evaluate under real conditions the potential of this all solid-state LSG-EC for flexible storage, the research team placed a sample under constant mechanical stress to analyze its performance, it had almost no effect on the performance of the device.
Kaner explains, “We attribute the high performance and durability to the high mechanical flexibility of the electrodes along with the interpenetrating network structure between the LSG electrodes and the gelled electrolyte. The electrolyte solidifies during the device assembly and acts like glue that holds the device components together.”
It looks as though the choices the team has made improves the mechanical integrity and increases the life cycle of the device even when tested under extreme conditions.
The press release seems to be driving to flexible and or portable device applications. There might be a bias or some unsaid circumstance, perhaps graphene costs or the electrolyte gel that suggests a smaller higher value first market.
That’s OK, its best to get to a market and build some experience before trying to hit the biggest market of all.
The UCLA team took a difficult challenge and answered with clever, innovative and simple solution. It will be a while before we know if the idea can get to mass production scale and at what prices.
Meanwhile – its sure looks good.
Comments
1 Comment so far


While the process of capacitor construction was loosely described, the process of electrical charging and discharging was not. Please clarify how you envision power sufficient to drive a vehicle could be stored and retrieved effectively.