Mar
13
Bit ‘O Gold Makes a Better Fuel Cell
March 13, 2012 | Leave a Comment
The study published in the Journal of the American Chemical Society reports the key is adding gold to the catalyst formation process to yield a more uniform crystal structure and the new crystal removes carbon monoxide from the reaction.
That’s the news – getting the carbon monoxide away. Platinum absorbs carbon monoxide in reactions involving fuel cells powered by organic materials like formic acid. Palladium breaks down over time. Both of these metals are very costly.
The Brown University created a triple-headed metallic nanoparticle that they say outperforms and outlasts all others at the anode end in formic-acid fuel-cell reactions. They report a 4-nanometer iron-platinum-gold nanoparticle (FePtAu), with a tetragonal crystal structure, generates higher current per unit of mass than any other nanoparticle catalyst tested. Better yet, Brown’s triple metal nanoparticle performs nearly as well after 13 hours as it did at the start.
That compares to another test built nanoparticle challenged under identical conditions that lost nearly 90% of its performance in just one-quarter of the time.
Shouheng Sun, chemistry professor at Brown and corresponding author on the paper said, “We’ve developed a formic acid fuel-cell catalyst that is the best to have been created and tested so far. It has good durability as well as good activity.”
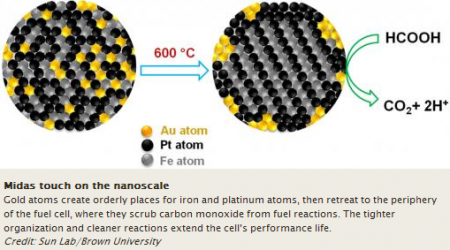
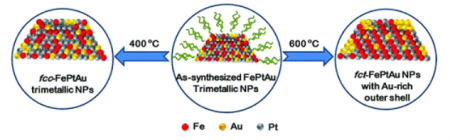
The bit of gold starts the positive effect right at the beginning, when the crystals are built. The gold acts as an atomic atom organizer of sorts, leading the iron and platinum atoms into neat, uniform layers within the nanoparticle. Then the gold atoms then exit the interior and bind to the outer surface of the nanoparticle assembly.
Gold is effective at ordering the iron and platinum atoms because the gold atoms create extra space within the nanoparticle sphere at the outset. When the gold atoms diffuse from the space upon heating, they create more room for the iron and platinum atoms to assemble themselves. As a bonus the gold creates the crystallization chemists want in the nanoparticle assembly at a lower temperature.
In operation the gold also removes carbon monoxide (CO) from the reaction by catalyzing its oxidation. Otherwise the carbon monoxide, which binds well to iron and platinum atoms, would gum up the reaction. By essentially scrubbing it from the reaction, gold improves the performance of the combined iron-platinum catalyst.
The team decided to try gold after reading in the literature that gold nanoparticles were effective at oxidizing carbon monoxide – so effective, in fact, that gold nanoparticles had been incorporated into the helmets of Japanese firefighters. Indeed, the Brown team’s triple-headed metallic nanoparticles worked just as well at removing CO in the oxidation of formic acid, although it is unclear specifically why.
In the study paper the Brown team highlights the importance of creating an ordered crystal structure for the nanoparticle catalyst. The gold additive helps researchers get a crystal structure called a “face-centered-tetragonal,” a four-sided shape in which iron and platinum atoms essentially are forced to occupy specific positions in the structure, creating more order. By imposing atomic order, the iron and platinum layers bind more tightly in the structure, thus making the assembly more stable and durable, essential to better-performing and longer-lasting catalysts.
The Brown team’s tests are extraordinary. The paper reports experiments showing the FePtAu catalyst reached 2809.9 mA/mg Pt (mass-activity, or current generated per milligram of platinum), “which is the highest among all NP (nanoparticle) catalysts ever reported.”. After 13 hours, the FePtAu nanoparticle has a mass activity of 2600mA/mg Pt, or 93% of its original performance value. The Brown team pointed out in comparison, the well-received platinum-bismuth nanoparticle has a mass activity of about 1720mA/mg Pt under identical experiments, and is four times less active when measured for durability.
The team also notes that other metals may be substituted for gold in the nanoparticle catalyst to improve the catalyst’s performance and durability. The team says in the study “This communication presents a new structure-control strategy to tune and optimize nanoparticle catalysis for fuel oxidations.”
This work is a breakthrough concept in crystal catalyst design. The ideas explored are going to have significant impact as the concept finds application across the catalyst field. The matter of scale will come up, but right out of the gate the team is addressing life expectancy with great results.
Who’s on the team? Inquiring headhunters will want to know.
Sen Zhang, a third-year graduate student in Sun’s lab, helped with the nanoparticle design and synthesis. Shaojun Guo, a postdoctoral fellow in Sun’s lab performed electrochemical oxidation experiments. Huiyuan Zhu, a second-year graduate student in Sun’s lab, synthesized the FePt nanoparticles and ran control experiments. The other contributing author is Dong Su from the Center for Functional Nanomaterials at Brookhaven National Laboratory, who analyzed the structure of the nanoparticle catalyst using the advanced electron microscopy facilities there.
Hold on – the funding was from ExxonMobil and the U.S. Department of Energy.
May the tech spread far and wide. Congratulations!