Jun
2
Enzyme Found That Makes Hydrocarbons
June 2, 2011 | 1 Comment
Utah State University (USU) biochemists Zhi-Yong Yang and Lance Seefeldt, along with colleague Dennis Dean of Virginia Tech, discovered a molybdenum nitrogenase enzyme capable of converting carbon monoxide into usable hydrocarbons and may lead to motor fuel molecules.
This is significant. Al Fin found the press release and your writer is posting now that the paper is up on the Internet. The paper “Molybdenum Nitrogenase Catalyzes the Reduction and Coupling of CO to Form Hydrocarbons,” is in the June 3, 2011 issue of The Journal of Biological Chemistry. The paper is available in a pdf file for free.
It seems the paper has gathered some noteworthy attention in the science community. The paper was selected as “Paper of the Week” by the journal’s editorial board, an honor bestowed on the top one percent of more than 6,600 manuscripts reviewed annually by the publication’s editors. In the “Paper of the Week” feature, Yang, a doctoral candidate mentored by Seefeldt, is highlighted as an up-and-coming researcher.
Seefeldt, professor in USU’s Department of Chemistry and Biochemistry understates the significance saying in the USU press release, “This is pretty profound, understanding this process paves the way for developing better ways of converting carbon monoxide, a toxic waste product of combustion, into transportation fuel and precursors for plastics – without the time and energy required for conventional extraction of fossil fuels.”
Coming up with carbon monoxide isn’t real hard, generally its just burn slowly without a lot of oxygen. One way to get lots is the Fischer-Tropsch or “FT” synthesis that can convert things like coal on up to light weight biomass.
Molybdenum nitrogenase isn’t one of the most common enzymes. Based on molybdenum and often called just “Moly,” for short, moly is a brittle, silver-gray metal found in soil and used in steel alloys. But it’s also found in small amounts in the human body, where it metabolizes certain amino acids, produces uric acid and helps to break down drugs and toxins.
When a substance is burned the CO yields an added benefit: it allows scientists to produce longer chain, double and triple-bond hydrocarbons, which provides a richer feedstock for production of refined transportation fuels. CO2 on the other hand has more oxygen, which makes reforming molecules more difficult. Extra oxygen around can be a problem.
Yang is a little more excited, saying, “Like many waste-to-energy processes, we’ve found we can produce such hydrocarbons as propane and butane from carbon monoxide, but using this process, we may have the potential to produce such transportation fuels as diesel and gasoline that are readily adaptable to today’s vehicles.”
Actually propane and butane would be just fine, they makes exceptionally good engine fuels because they burn so cleanly.
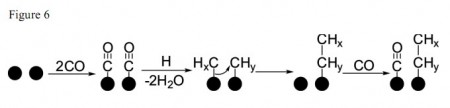
As the science sits today, the paper shows the USU pair has proven lab production of the alkanes ethane (C2H6) and propane (C3H8) and the alkenes ethylene (C2H4) and propene (C3H6) with the highest yield of ethylene, followed by propene, ethane, and propane.
That brings us back to the Fischer-Tropsch chemistry where finding catalysts and conditions that favor the formation of the higher value, longer chain hydrocarbons like propene and propane is a top research target.
The molybdenum nitrogenase enzyme offers an experimentally functioning system for examining the mechanical features that favor the production of longer chain hydrocarbons from CO. Continued research might drive development of small molecule metal complexes as catalysts useful in prototype and perhaps commercial scale reactions.
If, or likely when those catalyst are developed and installed into a Fischer-Tropsch reactor the product yields would be far more valuable and the value of the biomass or other feedstock would go further and produce more revenue.
Some observers believe that heating biomass with chemical processes to produce fuels isn’t competitive to biological digestion such as methanol or ethanol production processes. Both work, for now the biological path is in the lead. But the chemical potential hasn’t gotten full stride, and the USU team has just taken a very long and strong stride, indeed.
Comments
1 Comment so far


Good overview. This alternative approach is likely to be important if yields can be boosted.
Bacterial nitrogenases may not be tough enough catalysts to work with concentrated syngas for high yield production. Nanotechnologists may have to mimic the enzymes’ active site using inorganic materials.
Like you say, scalable economic production depends on the catalysts. I wonder what the best feedstocks will be initially. Natural gas? Coal? Biomass?