Nov
3
A New Way to Store the Sun’s Thermal Energy
November 3, 2010 | 8 Comments
Jeffrey Grossman, the Carl Richard Soderberg Associate Professor of Power Engineering in the Department of Materials Science and Engineering at MIT,Yosuke Kanai of Lawrence Livermore National Laboratory, Varadharajan Srinivasan of MIT’s Department of Materials Science and Engineering, and Steven Meier and Peter Vollhardt of the University of California, Berkeley are reporting they have mapped the behavior of a unique molecule to store and release heat – a kind of heat battery.
This is an unrealized concept. There have been two approaches to capturing the sun’s energy: photovoltaics, which turn the sunlight into electricity, or solar-thermal systems, which concentrate the sun’s heat and use it to boil water to turn a turbine, or use the heat directly for hot water or home heating. Both offer storage by either a chemical battery or in heated materials. But heated materials inevitably lose the heat to radiation even in the best insulation systems.
There is another approach the potential of which was seen decades ago, that was sidelined because nobody found a way to harness it in a practical and economical way. The approach is called thermo-chemical storage, in which solar energy is captured in the configuration of certain molecules, which can then release the energy on demand to produce usable heat. The heat-storing chemicals can remain stable for years.
Starting in the 1970s researchers sought the molecules that would accomplish the task. Twenty-six years passed in the search for a chemical that could reliably and reversibly switch between two states, absorbing sunlight to go into one state and then releasing heat when it reverted to the first state. Such a compound was discovered in 1996.
There is a major ‘but’ in this. The molecule included ruthenium, a very rare and expensive element, so it was impractical for widespread energy storage. Moreover, no one understood how the compound worked, which hindered efforts to find a cheaper variant.
Grossman and his team think they have the chemical; called fulvalene diruthenium (FD) mapped so understanding the behavior and function. Their report on the work was published on Oct. 20 in the journal Angewandte Chemie.
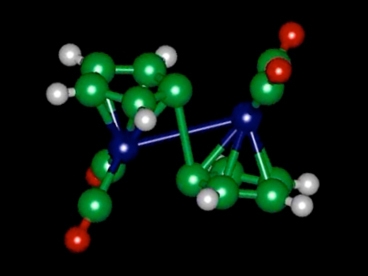
Fulvalene Diruthenium Molecule Diagram. Image Credit Jeffrey Grossman. Click image for the largest view.
FD undergoes a structural transformation when it absorbs sunlight, putting the molecule into a higher-energy state where it can remain stable indefinitely. Then, triggered by a small addition of heat or a catalyst, it snaps back to its original shape, releasing heat in the process. But the team found that the process is a bit more complicated than that.
Grossman explains, “It turns out there’s an intermediate step that plays a major role,” in this intermediate step, the molecule forms a semistable configuration partway between the two previously known states. “That was unexpected,” he said. The two-step process helps explain why the molecule is so stable, why the process is easily reversible and also why substituting other elements for ruthenium has not worked so far.
Grossman continues, in effect this makes it possible to produce a “rechargeable heat battery” that can repeatedly store and release heat gathered from sunlight or other sources. In principle, a battery made from fulvalene diruthenium, when its stored heat is released, “can get as hot as 200 degrees C, plenty hot enough to heat your home, or even to run an engine to produce electricity.” Compared to other approaches to solar energy, he said, “It takes many of the advantages of solar-thermal energy, but stores the heat in the form of a fuel. It’s reversible, and it’s stable over a long term. You can use it where you want, on demand. You could put the fuel in the sun, charge it up, then use the heat, and place the same fuel back in the sun to recharge.”
There you have it, thermo-chemical storage at extremely high prices.
Grossman is full of hope that much less costly molecules can be found. He said, “It’s my firm belief that as we understand what makes this material tick, we’ll find that there will be other materials” that will work the same way. The next step is to use a combination of simulation, chemical intuition, and databases of tens of millions of known molecules to look for other candidates that have structural similarities and might exhibit the same behavior.
The race is on.
The counter point comes from Roman Boulatov, assistant professor of chemistry at the University of Illinois at Urbana-Champaign, who said of the research that “its greatest accomplishment is to overcome significant challenges in quantum-chemical modeling of the reaction,” thus enabling the design of new types of molecules that could be used for energy storage. But he adds that other challenges remain: “Two other critical questions would have to be solved by other means, however. One, how easy is it to synthesize the best candidates? Second, what is a possible catalyst to trigger the release of the stored energy?”
Something intuitive suggests to this writer those matters will resolve over time.
Grossman plans to collaborate with MIT’s Daniel Nocera, the Henry Dreyfus Professor of Energy and Professor of Chemistry, to tackle such questions by applying the principles learned from this analysis in order to design new, inexpensive materials that exhibit this same reversible process. The tight coupling between computational materials design and experimental synthesis and validation, he said, should further accelerate the discovery of promising new candidate solar thermal fuels.
A not hot, but heat holding form of storage offers the imagination a wide array of applications. Cheap enough, heat could be stored and moved over time without a continuous leaking away. That’s a revolution in and of itself. The obvious use is heat collection through the summer for use in the winter. A major reduction in thermal collection facility would occur. The impact would be very substantial.
As basic research goes, this report must be 2010’s most significant achievement. Congratulations are in order for a discovery and exploration expedition now underway following a near 40-year wait.
Comments
8 Comments so far


Interesting – a solar heat battery, instead of a solar electricity generator, or a simple solar heat absorber. Of course photosynthesis is a kind of solar battery, too, but one which stores chemical reactive potential. I see this solar heat battery in the article as exemplifying two separate potential technologies – First, it is a way of storing heat without insulation (other than protection from a catalytic heat-trigger to release the stored heat), which itself is revolutionary, even if the external energy source is not solar. And second, it suggests a redefinition of what a battery can be, since we now see an example of solar energy being used to “flip” a molecule into a higher-energy state. Hypothetically, the released energy of some other, analogous, molecule doesn’t need to be heat – it could just as well be a change in chemical properties, resulting (for example) in an electrical battery that is charged by the sun but requires no intervening transducers to tap a stored electrical potential.
Sophisticated sun control saves energy and makes life easier…
We liked your article, so we (RambergMediaGroup) would be interested in hearing from you, our readers like it….
Pretty impressive post. I just came across your site and wanted to say I have really enjoyed reading your opinions. In whatever way I’ll be coming back and that I do hope you post again soon.
Hello, this is my first time i visit here. I found so many interesting in your blog especially on how to determine the topic. keep up the good work.
I’ve just started off a blog, the knowledge you give on this site has aided me extremely. Thank you for all your time & work.
I’ve been checking your blog for a while now, seems like everyday I learn something new 🙂 Thanks
Awesome post. I so good to see someone taking the time to share this information
I have learned some new things from your blog post. One other thing to I have observed is that in most cases, FSBO sellers will certainly reject you. Remember, they will prefer never to use your solutions. But if you maintain a gradual, professional relationship, offering assistance and staying in contact for around four to five weeks, you will usually have the capacity to win a business interview. From there, a house listing follows. Cheers