Sep
29
Make Hydrogen While You Drive
September 29, 2010 | 6 Comments
Some press releases go pretty far. One hopes the scientists proofread the text before it gets out. The University of Wisconsin says in its release about a low cost, low temperature hydrogen catalyst for purification, “Engineering researchers from Tufts University, the University of Wisconsin-Madison and Harvard University have demonstrated the low-temperature efficacy of an atomically dispersed platinum catalyst, which could be suitable for on-board hydrogen production in fuel-cell-powered vehicles of the future.”
That’s a very big suggestion.
The information is that new platinum-based catalyst is highly active and stable and can be an alternative to copper, which under certain conditions can ignite spontaneously.
The impressive university listed team members are led by Maria Flytzani-Stephanopoulos, Professor of Chemical and Biological Engineering at Tufts University School of Engineering, and Manos Mavrikakis, a UW-Madison Professor of Chemical and Biological Engineering. The research team published its findings in the Sept. 24 issue of the journal Science.
So far the team’s understanding of the structure and function of the new catalyst could help manufacturers design highly effective – but less costly – catalysts on standard, inexpensive supporting metal oxides.
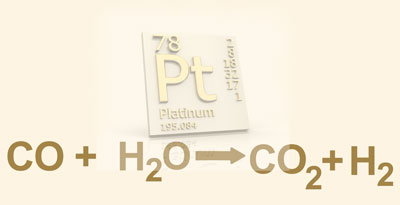
Here’s the problem. Fuel cells use electrochemical processes to convert hydrogen and oxygen into water, producing direct current that powers a motor. Automotive grade fuel cells require highly purified hydrogen, which is produced through a water-gas-shift reaction. This key step strips “residual” carbon monoxide from hydrogen generated through steam reforming of fossil fuels, such as natural gas. Water-gas-shift catalysts decrease the amount of carbon monoxide in hydrogen and increase the hydrogen content by harvesting hydrogen from water molecules.
Today’s commercial catalysts to purify hydrogen are copper-based, supported on zinc oxide and alumina. Because copper is pyrophoric, meaning it could spontaneously ignite when exposed to air, as air in fuel cell operation is relatively common, researchers have considered platinum as a substitute. But platinum is costly and, says Flytzani-Stephanopoulos, researchers must prepare it in very fine particles on more “exotic” supports, such as the rare-earth oxide ceria, which makes it effective for the low-temperature water-gas-shift reaction.
But while cerium is the most abundant of the “rare-earth” elements, this natural abundance occurs in just a few places around the world, and, says Mavrikakis, access to it may be limited for various reasons, including geopolitical motives.
A potential solution may come from the Tufts researchers initial discovery that sodium improves the platinum activity in the water-gas-shift reaction, which now can take place at low temperatures, even on inert materials like low cost silica. They carried out detailed structural studies and found extra active oxygen species on the surface that helped the platinum complete the reaction cycle. They also found that the sodium or potassium ions helped to stabilize the catalytic sites. In later experiments, they saw their catalyst perform as well as platinum on ceria.
Collaborator David Bell of Harvard University used atomic-resolution electron microscopy to view stabilized platinum clusters and atoms on the silica support – visual confirmation that the new catalyst operates like those on ceria supports.
In Wisconsin Mavrikakis’ team set out to understand why. The researchers drew on powerful computational resources, including the UW-Madison Division of Information Technology and the Center for High-Throughput Computing, as well as an ultrafast 10G data network, to model the new catalyst, atom by atom.
“There is no experimental way that you can look at the atoms ‘at work’ – that is, while the reaction is happening,” says Mavrikakis. “You need to start talking about individual atoms, which you can see with the highest-resolution electron microscopes – but not during the reaction. So you can only suggest that perhaps these atoms are active, but there is no way to substantiate it unless you put an atomic-scale quantum-mechanical model together and come up with a more realistic and well-founded suggestion about what is responsible for making this catalyst so active.”
The new catalyst contains only trace amounts of platinum, yet is robust and effective at low temperatures. Essentially, its structure is a series of small “clusters” comprising only a few atoms, each in a specific arrangement. Each cluster is composed of one or a few a platinum atoms surrounded by a mixture of oxygen, hydroxyl and potassium atoms and is “seated” on the standard aluminum or silica support.
The researchers explain the advance is important in part because, through a combination of experiments and first-principles theory, the work reveals a new type of active site for a specific, very important chemical reaction. “Most of the time, people are happy to say, ‘Well, we’ve found a material. It works for a given application,'” says Mavrikakis.
Flytzani-Stephanopoulos said the team took the next step to determine how and why the catalyst works. “If we want to move to the next stage with cheaper materials that are doing the specific chemical transformations, we need to understand the fundamentals.”
This could be a very significant step in the catalyst field. Platinum is usually the most expensive element for sale, even more expensive than gold. Getting equivalent work from trace amounts has to help the economies of a wide range of chemical activities in a modern economy.
A vehicle fueling with say natural gas and some water powering a fuel cell is a still huge engineering exercise. But by no means is it impossible, it’s just that no one has set out to build such a system as the platinum catalyst costs just kill the idea.
Maybe now it could be a practical idea. It will be interesting to see how the efficiency stacks up, and the drivability issues turn out. Perhaps compressed natural gas, a bulky fuel product at very high efficiencies would be a base for a competitive personal transport vehicle. A hydrogen forming, self-cleaning, fuel cell powered vehicle is a project that now seems feasible – surely closer to reality.
Comments
6 Comments so far


[…] rest is here: Make Hydrogen While You Drive | New Energy and Fuel By admin | category: TUFTS University | tags: fully-processed, key-role, […]
Interesting read, perhaps the best article iv’e browse today. We learn everyday cheers to you!
I’ve been checking your blog for a while now, seems like everyday I learn something new 🙂 Thanks
Hello, this is my first time i visit here. I found so many interesting in your blog especially on how to determine the topic. keep up the good work.
Intriguing post. I have been searching for some good resources for solar panels and discovered your blog. Planning to bookmark this one!
Good! Thank you! I always wanted to write in my site something like that. Can I take part of your post to my blog?